+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-22408 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | 1:1 cGAS-nucleosome complex | |||||||||
 Map data Map data | 1:1 cGAS-NCP map | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | cGAS / nucleosome / cyclic GMP-AMP synthase / DNA Binding Protein-DNA-Transferase complex | |||||||||
| Function / homology |  Function and homology information Function and homology informationregulation of type I interferon production / 2',3'-cyclic GMP-AMP synthase activity / cyclic GMP-AMP synthase / paracrine signaling / poly-ADP-D-ribose modification-dependent protein binding / regulation of immunoglobulin production / negative regulation of DNA repair / cGAS/STING signaling pathway / regulation of T cell activation / cGMP-mediated signaling ...regulation of type I interferon production / 2',3'-cyclic GMP-AMP synthase activity / cyclic GMP-AMP synthase / paracrine signaling / poly-ADP-D-ribose modification-dependent protein binding / regulation of immunoglobulin production / negative regulation of DNA repair / cGAS/STING signaling pathway / regulation of T cell activation / cGMP-mediated signaling / negative regulation of cGAS/STING signaling pathway / cellular response to exogenous dsRNA / positive regulation of type I interferon production / regulation of immune response / negative regulation of double-strand break repair via homologous recombination / negative regulation of megakaryocyte differentiation / protein localization to CENP-A containing chromatin / Chromatin modifying enzymes / Replacement of protamines by nucleosomes in the male pronucleus / cAMP-mediated signaling / CENP-A containing nucleosome / nucleosome binding / Packaging Of Telomere Ends / Recognition and association of DNA glycosylase with site containing an affected purine / Cleavage of the damaged purine / positive regulation of defense response to virus by host / Deposition of new CENPA-containing nucleosomes at the centromere / phosphatidylinositol-4,5-bisphosphate binding / Recognition and association of DNA glycosylase with site containing an affected pyrimidine / Cleavage of the damaged pyrimidine / telomere organization / Meiotic synapsis / Inhibition of DNA recombination at telomere / Interleukin-7 signaling / RNA Polymerase I Promoter Opening / activation of innate immune response / Assembly of the ORC complex at the origin of replication / Regulation of endogenous retroelements by the Human Silencing Hub (HUSH) complex / SUMOylation of chromatin organization proteins / DNA methylation / Condensation of Prophase Chromosomes / Chromatin modifications during the maternal to zygotic transition (MZT) / HCMV Late Events / SIRT1 negatively regulates rRNA expression / ERCC6 (CSB) and EHMT2 (G9a) positively regulate rRNA expression / innate immune response in mucosa / PRC2 methylates histones and DNA / Regulation of endogenous retroelements by KRAB-ZFP proteins / Defective pyroptosis / determination of adult lifespan / Regulation of endogenous retroelements by Piwi-interacting RNAs (piRNAs) / HDACs deacetylate histones / Nonhomologous End-Joining (NHEJ) / RNA Polymerase I Promoter Escape / Transcriptional regulation by small RNAs / Formation of the beta-catenin:TCF transactivating complex / molecular condensate scaffold activity / RUNX1 regulates genes involved in megakaryocyte differentiation and platelet function / Activated PKN1 stimulates transcription of AR (androgen receptor) regulated genes KLK2 and KLK3 / G2/M DNA damage checkpoint / HDMs demethylate histones / NoRC negatively regulates rRNA expression / DNA Damage/Telomere Stress Induced Senescence / B-WICH complex positively regulates rRNA expression / PKMTs methylate histone lysines / Meiotic recombination / Pre-NOTCH Transcription and Translation / Metalloprotease DUBs / RMTs methylate histone arginines / Activation of anterior HOX genes in hindbrain development during early embryogenesis / HCMV Early Events / Transcriptional regulation of granulopoiesis / heterochromatin formation / positive regulation of cellular senescence / nucleosome assembly / structural constituent of chromatin / antimicrobial humoral immune response mediated by antimicrobial peptide / UCH proteinases / antibacterial humoral response / nucleosome / E3 ubiquitin ligases ubiquitinate target proteins / Recruitment and ATM-mediated phosphorylation of repair and signaling proteins at DNA double strand breaks / chromatin organization / RUNX1 regulates transcription of genes involved in differentiation of HSCs / site of double-strand break / HATs acetylate histones / Factors involved in megakaryocyte development and platelet production / Processing of DNA double-strand break ends / MLL4 and MLL3 complexes regulate expression of PPARG target genes in adipogenesis and hepatic steatosis / Senescence-Associated Secretory Phenotype (SASP) / double-stranded DNA binding / Oxidative Stress Induced Senescence / defense response to virus / Estrogen-dependent gene expression / chromosome, telomeric region / nuclear body / defense response to Gram-positive bacterium / Ub-specific processing proteases / protein heterodimerization activity / Amyloid fiber formation Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) / Homo sapiens (human) /  | |||||||||
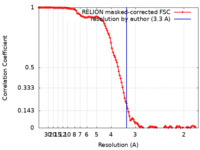
| Method | single particle reconstruction / cryo EM / Resolution: 3.3 Å | |||||||||
 Authors Authors | Boyer JA / Spangler CJ | |||||||||
| Funding support |  United States, 2 items United States, 2 items
| |||||||||
 Citation Citation |  Journal: Science / Year: 2020 Journal: Science / Year: 2020Title: Structural basis of nucleosome-dependent cGAS inhibition. Authors: Joshua A Boyer / Cathy J Spangler / Joshua D Strauss / Andrew P Cesmat / Pengda Liu / Robert K McGinty / Qi Zhang /  Abstract: Cyclic guanosine monophosphate (GMP)-adenosine monophosphate (AMP) synthase (cGAS) recognizes cytosolic foreign or damaged DNA to activate the innate immune response to infection, inflammatory ...Cyclic guanosine monophosphate (GMP)-adenosine monophosphate (AMP) synthase (cGAS) recognizes cytosolic foreign or damaged DNA to activate the innate immune response to infection, inflammatory diseases, and cancer. By contrast, cGAS reactivity against self-DNA in the nucleus is suppressed by chromatin tethering. We report a 3.3-angstrom-resolution cryo-electron microscopy structure of cGAS in complex with the nucleosome core particle. The structure reveals that cGAS uses two conserved arginines to anchor to the nucleosome acidic patch. The nucleosome-binding interface exclusively occupies the strong double-stranded DNA (dsDNA)-binding surface on cGAS and sterically prevents cGAS from oligomerizing into the functionally active 2:2 cGAS-dsDNA state. These findings provide a structural basis for how cGAS maintains an inhibited state in the nucleus and further exemplify the role of the nucleosome in regulating diverse nuclear protein functions. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_22408.map.gz emd_22408.map.gz | 10.2 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-22408-v30.xml emd-22408-v30.xml emd-22408.xml emd-22408.xml | 20.9 KB 20.9 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_22408_fsc.xml emd_22408_fsc.xml | 11.6 KB | Display |  FSC data file FSC data file |
| Images |  emd_22408.png emd_22408.png | 150.1 KB | ||
| Filedesc metadata |  emd-22408.cif.gz emd-22408.cif.gz | 7.1 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-22408 http://ftp.pdbj.org/pub/emdb/structures/EMD-22408 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-22408 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-22408 | HTTPS FTP |
-Validation report
| Summary document |  emd_22408_validation.pdf.gz emd_22408_validation.pdf.gz | 399.1 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_22408_full_validation.pdf.gz emd_22408_full_validation.pdf.gz | 398.7 KB | Display | |
| Data in XML |  emd_22408_validation.xml.gz emd_22408_validation.xml.gz | 12.1 KB | Display | |
| Data in CIF |  emd_22408_validation.cif.gz emd_22408_validation.cif.gz | 16 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-22408 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-22408 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-22408 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-22408 | HTTPS FTP |
-Related structure data
| Related structure data |  7jo9MC  7joaC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_22408.map.gz / Format: CCP4 / Size: 137.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_22408.map.gz / Format: CCP4 / Size: 137.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | 1:1 cGAS-NCP map | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.91 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : 1:1 cGAS-nucleosome complex
| Entire | Name: 1:1 cGAS-nucleosome complex |
|---|---|
| Components |
|
-Supramolecule #1: 1:1 cGAS-nucleosome complex
| Supramolecule | Name: 1:1 cGAS-nucleosome complex / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#7 / Details: mouse cGAS bound to the nucleosome in a 1:1 ratio |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 250 KDa |
-Macromolecule #1: Histone H3.2
| Macromolecule | Name: Histone H3.2 / type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 15.421101 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MARTKQTARK STGGKAPRKQ LATKAARKSA PATGGVKKPH RYRPGTVALR EIRRYQKSTE LLIRKLPFQR LVREIAQDFK TDLRFQSSA VMALQEASEA YLVGLFEDTN LCAIHAKRVT IMPKDIQLAR RIRGERA UniProtKB: Histone H3.2 |
-Macromolecule #2: Histone H4
| Macromolecule | Name: Histone H4 / type: protein_or_peptide / ID: 2 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 11.394426 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MSGRGKGGKG LGKGGAKRHR KVLRDNIQGI TKPAIRRLAR RGGVKRISGL IYEETRGVLK VFLENVIRDA VTYTEHAKRK TVTAMDVVY ALKRQGRTLY GFGG UniProtKB: Histone H4 |
-Macromolecule #3: Histone H2A type 1
| Macromolecule | Name: Histone H2A type 1 / type: protein_or_peptide / ID: 3 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 13.990342 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: SGRGKQGGKA RAKAKTRSSR AGLQFPVGRV HRLLRKGNYA ERVGAGAPVY LAAVLEYLTA EILELAGNAA RDNKKTRIIP RHLQLAIRN DEELNKLLGK VTIAQGGVLP NIQAVLLPKK TESHHKAKGK UniProtKB: Histone H2A type 1 |
-Macromolecule #4: Histone H2B type 1-C/E/F/G/I
| Macromolecule | Name: Histone H2B type 1-C/E/F/G/I / type: protein_or_peptide / ID: 4 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 13.806018 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: PEPAKSAPAP KKGSKKAVTK AQKKDGKKRK RSRKESYSVY VYKVLKQVHP DTGISSKAMG IMNSFVNDIF ERIAGEASRL AHYNKRSTI TSREIQTAVR LLLPGELAKH AVSEGTKAVT KYTSSK UniProtKB: Histone H2B type 1-C/E/F/G/I |
-Macromolecule #7: Cyclic GMP-AMP synthase
| Macromolecule | Name: Cyclic GMP-AMP synthase / type: protein_or_peptide / ID: 7 / Number of copies: 1 / Enantiomer: LEVO / EC number: cyclic GMP-AMP synthase |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 42.984664 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: GSRKEPDKLK KVLDKLRLKR KDISEAAETV NKVVERLLRR MQKRESEFKG VEQLNTGSYY EHVKISAPNE FDVMFKLEVP RIELQEYYE TGAFYLVKFK RIPRGNPLSH FLEGEVLSAT KMLSKFRKII KEEVKEIKDI DVSVEKEKPG SPAVTLLIRN P EEISVDII ...String: GSRKEPDKLK KVLDKLRLKR KDISEAAETV NKVVERLLRR MQKRESEFKG VEQLNTGSYY EHVKISAPNE FDVMFKLEVP RIELQEYYE TGAFYLVKFK RIPRGNPLSH FLEGEVLSAT KMLSKFRKII KEEVKEIKDI DVSVEKEKPG SPAVTLLIRN P EEISVDII LALESKGSWP ISTKEGLPIQ GWLGTKVRTN LRREPFYLVP KNAKDGNSFQ GETWRLSFSH TEKYILNNHG IE KTCCESS GAKCCRKECL KLMKYLLEQL KKEFQELDAF CSYHVKTAIF HMWTQDPQDS QWDPRNLSSC FDKLLAFFLE CLR TEKLDH YFIPKFNLFS QELIDRKSKE FLSKKIEYER NNGFPIFDKL UniProtKB: Cyclic GMP-AMP synthase |
-Macromolecule #5: DNA (145-MER)
| Macromolecule | Name: DNA (145-MER) / type: dna / ID: 5 / Number of copies: 1 / Classification: DNA |
|---|---|
| Source (natural) | Organism: synthetic construct (others) |
| Molecular weight | Theoretical: 45.610043 KDa |
| Sequence | String: (DA)(DT)(DC)(DG)(DG)(DA)(DT)(DG)(DT)(DA) (DT)(DA)(DT)(DA)(DT)(DC)(DT)(DG)(DA)(DC) (DA)(DC)(DG)(DT)(DG)(DC)(DC)(DT)(DG) (DG)(DA)(DG)(DA)(DC)(DT)(DA)(DG)(DG)(DG) (DA) (DG)(DT)(DA)(DA)(DT)(DC) ...String: (DA)(DT)(DC)(DG)(DG)(DA)(DT)(DG)(DT)(DA) (DT)(DA)(DT)(DA)(DT)(DC)(DT)(DG)(DA)(DC) (DA)(DC)(DG)(DT)(DG)(DC)(DC)(DT)(DG) (DG)(DA)(DG)(DA)(DC)(DT)(DA)(DG)(DG)(DG) (DA) (DG)(DT)(DA)(DA)(DT)(DC)(DC)(DC) (DC)(DT)(DT)(DG)(DG)(DC)(DG)(DG)(DT)(DT) (DA)(DA) (DA)(DA)(DC)(DG)(DC)(DG)(DG) (DG)(DG)(DG)(DA)(DC)(DA)(DG)(DC)(DG)(DC) (DG)(DT)(DA) (DC)(DG)(DT)(DG)(DC)(DG) (DT)(DT)(DT)(DA)(DA)(DG)(DC)(DG)(DG)(DT) (DG)(DC)(DT)(DA) (DG)(DA)(DG)(DC)(DT) (DG)(DT)(DC)(DT)(DA)(DC)(DG)(DA)(DC)(DC) (DA)(DA)(DT)(DT)(DG) (DA)(DG)(DC)(DG) (DG)(DC)(DC)(DT)(DC)(DG)(DG)(DC)(DA)(DC) (DC)(DG)(DG)(DG)(DA)(DT) (DT)(DC)(DT) (DC)(DG)(DA)(DT) |
-Macromolecule #6: DNA (145-MER)
| Macromolecule | Name: DNA (145-MER) / type: dna / ID: 6 / Number of copies: 1 / Classification: DNA |
|---|---|
| Source (natural) | Organism: synthetic construct (others) |
| Molecular weight | Theoretical: 45.13877 KDa |
| Sequence | String: (DA)(DT)(DC)(DG)(DA)(DG)(DA)(DA)(DT)(DC) (DC)(DC)(DG)(DG)(DT)(DG)(DC)(DC)(DG)(DA) (DG)(DG)(DC)(DC)(DG)(DC)(DT)(DC)(DA) (DA)(DT)(DT)(DG)(DG)(DT)(DC)(DG)(DT)(DA) (DG) (DA)(DC)(DA)(DG)(DC)(DT) ...String: (DA)(DT)(DC)(DG)(DA)(DG)(DA)(DA)(DT)(DC) (DC)(DC)(DG)(DG)(DT)(DG)(DC)(DC)(DG)(DA) (DG)(DG)(DC)(DC)(DG)(DC)(DT)(DC)(DA) (DA)(DT)(DT)(DG)(DG)(DT)(DC)(DG)(DT)(DA) (DG) (DA)(DC)(DA)(DG)(DC)(DT)(DC)(DT) (DA)(DG)(DC)(DA)(DC)(DC)(DG)(DC)(DT)(DT) (DA)(DA) (DA)(DC)(DG)(DC)(DA)(DC)(DG) (DT)(DA)(DC)(DG)(DC)(DG)(DC)(DT)(DG)(DT) (DC)(DC)(DC) (DC)(DC)(DG)(DC)(DG)(DT) (DT)(DT)(DT)(DA)(DA)(DC)(DC)(DG)(DC)(DC) (DA)(DA)(DG)(DG) (DG)(DG)(DA)(DT)(DT) (DA)(DC)(DT)(DC)(DC)(DC)(DT)(DA)(DG)(DT) (DC)(DT)(DC)(DC)(DA) (DG)(DG)(DC)(DA) (DC)(DG)(DT)(DG)(DT)(DC)(DA)(DG)(DA)(DT) (DA)(DT)(DA)(DT)(DA)(DC) (DA)(DT)(DC) (DC)(DG)(DA)(DT) |
-Macromolecule #8: ZINC ION
| Macromolecule | Name: ZINC ION / type: ligand / ID: 8 / Number of copies: 1 / Formula: ZN |
|---|---|
| Molecular weight | Theoretical: 65.409 Da |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 1 mg/mL | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.5 Component:
| ||||||||||||
| Grid | Model: Quantifoil / Material: COPPER / Mesh: 400 / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 30 sec. / Details: Instrument: Pelco easiGlow | ||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 95 % / Chamber temperature: 293 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TALOS ARCTICA |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Number grids imaged: 1 / Number real images: 2100 / Average electron dose: 53.0 e/Å2 |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Talos Arctica / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller

























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)