[English] 日本語
 Yorodumi
Yorodumi- EMDB-21396: Porcine epidemic diarrhea virus (PEDV) spike protein with N264D m... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-21396 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Porcine epidemic diarrhea virus (PEDV) spike protein with N264D mutation, expressed in 293F cells and negatively stained | |||||||||
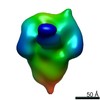
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
| Biological species |  Porcine epidemic diarrhea virus Porcine epidemic diarrhea virus | |||||||||
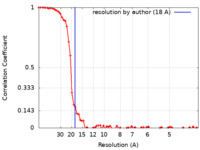
| Method | single particle reconstruction / negative staining / Resolution: 18.0 Å | |||||||||
 Authors Authors | Kirchdoerfer RN / Martini O / Ward AB | |||||||||
| Funding support |  United States, 2 items United States, 2 items
| |||||||||
 Citation Citation |  Journal: Structure / Year: 2021 Journal: Structure / Year: 2021Title: Structure and immune recognition of the porcine epidemic diarrhea virus spike protein. Authors: Robert N Kirchdoerfer / Mahesh Bhandari / Olnita Martini / Leigh M Sewall / Sandhya Bangaru / Kyoung-Jin Yoon / Andrew B Ward /  Abstract: Porcine epidemic diarrhea virus (PEDV) is an alphacoronavirus responsible for significant morbidity and mortality in pigs. A key determinant of viral tropism and entry, the PEDV spike protein is a ...Porcine epidemic diarrhea virus (PEDV) is an alphacoronavirus responsible for significant morbidity and mortality in pigs. A key determinant of viral tropism and entry, the PEDV spike protein is a key target for the host antibody response and a good candidate for a protein-based vaccine immunogen. We used electron microscopy to evaluate the PEDV spike structure, as well as pig polyclonal antibody responses to viral infection. The structure of the PEDV spike reveals a configuration similar to that of HuCoV-NL63. Several PEDV protein-protein interfaces are mediated by non-protein components, including a glycan at Asn264 and two bound palmitoleic acid molecules. The polyclonal antibody response to PEDV infection shows a dominance of epitopes in the S1 region. This structural and immune characterization provides insights into coronavirus spike stability determinants and explores the immune landscape of viral spike proteins. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_21396.map.gz emd_21396.map.gz | 13.1 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-21396-v30.xml emd-21396-v30.xml emd-21396.xml emd-21396.xml | 17.3 KB 17.3 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_21396_fsc.xml emd_21396_fsc.xml | 9 KB | Display |  FSC data file FSC data file |
| Images |  emd_21396.png emd_21396.png | 78.4 KB | ||
| Masks |  emd_21396_msk_1.map emd_21396_msk_1.map | 27 MB |  Mask map Mask map | |
| Others |  emd_21396_half_map_1.map.gz emd_21396_half_map_1.map.gz emd_21396_half_map_2.map.gz emd_21396_half_map_2.map.gz | 25 MB 25 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-21396 http://ftp.pdbj.org/pub/emdb/structures/EMD-21396 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-21396 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-21396 | HTTPS FTP |
-Validation report
| Summary document |  emd_21396_validation.pdf.gz emd_21396_validation.pdf.gz | 430 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_21396_full_validation.pdf.gz emd_21396_full_validation.pdf.gz | 429.5 KB | Display | |
| Data in XML |  emd_21396_validation.xml.gz emd_21396_validation.xml.gz | 13.1 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-21396 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-21396 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-21396 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-21396 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_21396.map.gz / Format: CCP4 / Size: 27 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_21396.map.gz / Format: CCP4 / Size: 27 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 2.05 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Mask #1
| File |  emd_21396_msk_1.map emd_21396_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_21396_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_21396_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Porcine epidemic diarrhea virus spike protein
| Entire | Name: Porcine epidemic diarrhea virus spike protein |
|---|---|
| Components |
|
-Supramolecule #1: Porcine epidemic diarrhea virus spike protein
| Supramolecule | Name: Porcine epidemic diarrhea virus spike protein / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all Details: Recombinantly expressed in 293F cells using transient transfection |
|---|---|
| Source (natural) | Organism:  Porcine epidemic diarrhea virus / Strain: 13-019349 Porcine epidemic diarrhea virus / Strain: 13-019349 |
| Recombinant expression | Organism:  Homo sapiens (human) / Recombinant strain: 293F / Recombinant plasmid: pcDNA3.4 Homo sapiens (human) / Recombinant strain: 293F / Recombinant plasmid: pcDNA3.4 |
| Molecular weight | Experimental: 600 KDa |
-Macromolecule #1: Porcine epidemic diarrhea virus (PEDV) spike protein
| Macromolecule | Name: Porcine epidemic diarrhea virus (PEDV) spike protein / type: protein_or_peptide / ID: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Porcine epidemic diarrhea virus Porcine epidemic diarrhea virus |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: LPQDVTRCSA NTNFRRFFSK FNVQAPAVVV LGGYLPIGEN QG VNSTWYC AGQHPTASGV HGIFVSHIRG GHGFEIGISQ EPFDPSGYQL YLHKATNGNT NAT ARLRIC QFPSIKTLGP TANNDVTTGR NCLFNKAIPA HMSEHSVVGI TWDNDRVTVF SDKI YYFYF ...String: LPQDVTRCSA NTNFRRFFSK FNVQAPAVVV LGGYLPIGEN QG VNSTWYC AGQHPTASGV HGIFVSHIRG GHGFEIGISQ EPFDPSGYQL YLHKATNGNT NAT ARLRIC QFPSIKTLGP TANNDVTTGR NCLFNKAIPA HMSEHSVVGI TWDNDRVTVF SDKI YYFYF KNDWSRVATK CYNSGGCAMQ YVYEPTYYML NVTSAGEDGI SYQPCTANCI GYAAN VFAT EPNGHIPEGF SFNNWFLLSD DSTLVHGKVV SNQPLLVNCL LAIPKIYGLG QFFSFN QTI DGVCNGAAVQ RAPEALRFNI NDISVILAEG SIVLHTALGT NFSFVCSNSS NPHLATF AI PLGATQVPYY CFLKVDTYNS TVYKFLAVLP PTVREIVITK YGDVYVNGFG YLHLGLLD A VTINFTGHGT DDDVSGFWTI ASTNFVDALI EVQGTAIQRI LYCDDPVSQL KCSQVAFDL DDGFYTISSR NLLSHEQPIS FVTLPSFNDH SFVNITVSAS FGGHSGANLI ASDTTINGFS SFCVDTRQF TISLFYNVTN SYGYVSKSQD SNCPFTLQSV NDYLSFSKFC VSTSLLASAC T IDLFGYPE FGSGVKFTSL YFQFTKGELI TGTPKPFEGV TDVSFMTLDV CTKYTIYGFK GE GIITLTN SSFLAGVYYT SDSGQLLAFK NVTSGAVYSV TPCSFSEQAA YVDDDIVGVI SSL SSSTFN STRELPGFFY HSNDGSNCTE PVLVYSNIGV CKSGSIGYVP SQSGQVKIAP TVTG NISIP TNFSMSIRTE YLQLYNTPVS VDCATYVCNG NSRCKQLLTQ YTAACKTIES ALQLS ARLE SVEVNSMLTI SDEALQLATI SSFNGDGYNF TNVLGVSVYD PASGRVVQKR SFIEDL LFN KVVTNGLGTV DEDYKRCSNG RSVADLVCAQ YYSGVMVLPG VVDAEKLHMY SASLIGG MV LGGFTSAAAL PFSYAVQARL NYLALQTDVL QRNQQLLAES FNSAIGNITS AFESVKEA I SQTSKGLNTV AHALTKVQEV VNSQGAALTQ LTVQLQHNFQ AISSSIDDIY SRLDILSAD AQVDRLITGR LSALNAFVAQ TLTKYTEVQA SRKLAQQKVN ECVKSQSQRY GFCGGDGEHI FSLVQAAPQ GLLFLHTVLV PSDFVDVIAI AGLCVNDEIA LTLREPGLVL FTHELQNHTA T EYFVSSRR MFEPRKPTVS DFVQIESCVV TYVNLTRDQL PDVIPDYIDV NKTLDEILAS LP NRTGPSL PLDVFNATYL NLTGEIADLE QRSESLRNTT EELQSLIYNI NNTLVDLEWL NRV ETGSGY IPEAPRDGQA YVRKDGEWVL LSTFLENLYF QGGHHHHHHA WSHPQFEK |
-Experimental details
-Structure determination
| Method | negative staining |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.03 mg/mL | ||||||
|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.4 Component:
| ||||||
| Staining | Type: NEGATIVE / Material: Uranyl formate | ||||||
| Grid | Support film - Material: CARBON / Support film - topology: CONTINUOUS / Details: unspecified |
- Electron microscopy
Electron microscopy
| Microscope | FEI TECNAI SPIRIT |
|---|---|
| Image recording | Film or detector model: TVIPS TEMCAM-F416 (4k x 4k) / Detector mode: COUNTING / Digitization - Frames/image: 1-64 / Number grids imaged: 1 / Average exposure time: 0.6 sec. / Average electron dose: 25.0 e/Å2 |
| Electron beam | Acceleration voltage: 120 kV / Electron source: LAB6 |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus min: 1.0 µm |
| Sample stage | Specimen holder model: OTHER |
| Experimental equipment |  Model: Tecnai Spirit / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller


 UCSF Chimera
UCSF Chimera



















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)