+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Tribolium castaneum hexamerin 2 | |||||||||||||||
 Map data Map data | hexamerin main map | |||||||||||||||
 Sample Sample |
| |||||||||||||||
 Keywords Keywords | hexamerin / tribolium castaneum / storage protein / UNKNOWN FUNCTION | |||||||||||||||
| Function / homology |  Function and homology information Function and homology informationArthropod hemocyanins / insect LSPs signature 2. / Hemocyanin, N-terminal / Hemocyanin, N-terminal domain superfamily / Hemocyanin, all-alpha domain / Hemocyanin, C-terminal domain superfamily / Hemocyanin/hexamerin middle domain / Hemocyanin, C-terminal / Hemocyanin, copper containing domain / Hemocyanin, ig-like domain / Hemocyanin/hexamerin ...Arthropod hemocyanins / insect LSPs signature 2. / Hemocyanin, N-terminal / Hemocyanin, N-terminal domain superfamily / Hemocyanin, all-alpha domain / Hemocyanin, C-terminal domain superfamily / Hemocyanin/hexamerin middle domain / Hemocyanin, C-terminal / Hemocyanin, copper containing domain / Hemocyanin, ig-like domain / Hemocyanin/hexamerin / Di-copper centre-containing domain superfamily / Immunoglobulin E-set Similarity search - Domain/homology | |||||||||||||||
| Biological species |  | |||||||||||||||
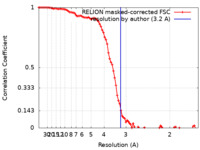
| Method | single particle reconstruction / cryo EM / Resolution: 3.2 Å | |||||||||||||||
 Authors Authors | Valentova L / Fuzik T / Plevka P | |||||||||||||||
| Funding support |  Czech Republic, European Union, 4 items Czech Republic, European Union, 4 items
| |||||||||||||||
 Citation Citation |  Journal: Acta Crystallogr D Struct Biol / Year: 2022 Journal: Acta Crystallogr D Struct Biol / Year: 2022Title: Polyelectrolyte coating of cryo-EM grids improves lateral distribution and prevents aggregation of macromolecules. Authors: Dominik Hrebík / Mária Gondová / Lucie Valentová / Tibor Füzik / Antonín Přidal / Jiří Nováček / Pavel Plevka /  Abstract: Cryo-electron microscopy (cryo-EM) is one of the primary methods used to determine the structures of macromolecules and their complexes. With the increased availability of cryo-electron microscopes, ...Cryo-electron microscopy (cryo-EM) is one of the primary methods used to determine the structures of macromolecules and their complexes. With the increased availability of cryo-electron microscopes, the preparation of high-quality samples has become a bottleneck in the cryo-EM structure-determination pipeline. Macromolecules can be damaged during the purification or preparation of vitrified samples for cryo-EM, making them prone to binding to the grid support, to aggregation or to the adoption of preferential orientations at the air-water interface. Here, it is shown that coating cryo-EM grids with a negatively charged polyelectrolyte, such as single-stranded DNA, before applying the sample reduces the aggregation of macromolecules and improves their distribution. The single-stranded DNA-coated grids enabled the determination of high-resolution structures from samples that aggregated on conventional grids. The polyelectrolyte coating reduces the diffusion of macromolecules and thus may limit the negative effects of the contact of macromolecules with the grid support and blotting paper, as well as of the shear forces on macromolecules during grid blotting. Coating grids with polyelectrolytes can readily be employed in any laboratory dealing with cryo-EM sample preparation, since it is fast, simple, inexpensive and does not require specialized equipment. | |||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_14679.map.gz emd_14679.map.gz | 6.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-14679-v30.xml emd-14679-v30.xml emd-14679.xml emd-14679.xml | 20.8 KB 20.8 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_14679_fsc.xml emd_14679_fsc.xml | 9.2 KB | Display |  FSC data file FSC data file |
| Images |  emd_14679.png emd_14679.png | 187.9 KB | ||
| Filedesc metadata |  emd-14679.cif.gz emd-14679.cif.gz | 6.2 KB | ||
| Others |  emd_14679_additional_1.map.gz emd_14679_additional_1.map.gz emd_14679_half_map_1.map.gz emd_14679_half_map_1.map.gz emd_14679_half_map_2.map.gz emd_14679_half_map_2.map.gz | 535.9 KB 49.8 MB 49.8 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-14679 http://ftp.pdbj.org/pub/emdb/structures/EMD-14679 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-14679 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-14679 | HTTPS FTP |
-Validation report
| Summary document |  emd_14679_validation.pdf.gz emd_14679_validation.pdf.gz | 1.1 MB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_14679_full_validation.pdf.gz emd_14679_full_validation.pdf.gz | 1.1 MB | Display | |
| Data in XML |  emd_14679_validation.xml.gz emd_14679_validation.xml.gz | 16.6 KB | Display | |
| Data in CIF |  emd_14679_validation.cif.gz emd_14679_validation.cif.gz | 21 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-14679 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-14679 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-14679 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-14679 | HTTPS FTP |
-Related structure data
| Related structure data |  7ze1MC  7zfwC  7zg7C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_14679.map.gz / Format: CCP4 / Size: 23 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_14679.map.gz / Format: CCP4 / Size: 23 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | hexamerin main map | ||||||||||||||||||||
| Voxel size | X=Y=Z: 0.822 Å | ||||||||||||||||||||
| Density |
| ||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Additional map: mask of the half maps
| File | emd_14679_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | mask of the half maps | ||||||||||||
| Projections & Slices |
| ||||||||||||
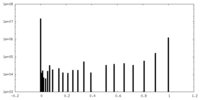
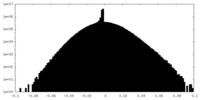
| Density Histograms |
-Half map: #2
| File | emd_14679_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
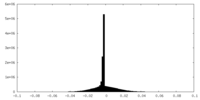
| Density Histograms |
-Half map: #1
| File | emd_14679_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
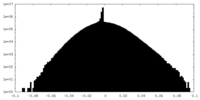
| Density Histograms |
- Sample components
Sample components
-Entire : Hexamerin 2
| Entire | Name: Hexamerin 2 |
|---|---|
| Components |
|
-Supramolecule #1: Hexamerin 2
| Supramolecule | Name: Hexamerin 2 / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all / Details: hexamerin isolated from Tribolium castaneum pupae |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 520 KDa |
-Macromolecule #1: Larval serum protein 1 gamma chain-like Protein
| Macromolecule | Name: Larval serum protein 1 gamma chain-like Protein / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 84.656922 KDa |
| Sequence | String: MRFILVAVLA GLCAVSAIST SESSNYSQQQ RDVWRLFKYI NQPSYYKDHV EIAHSYYFYD HASNYAKHEV VEEFYRYFKY DTFLQRGEI FSVFHGEHLK QAIALFKLFY YANDFDTFYK TAVWARQHVN EGMFLYAFSV ALIHRPDTYY FSLPPIYEIY P HYFYNYEV ...String: MRFILVAVLA GLCAVSAIST SESSNYSQQQ RDVWRLFKYI NQPSYYKDHV EIAHSYYFYD HASNYAKHEV VEEFYRYFKY DTFLQRGEI FSVFHGEHLK QAIALFKLFY YANDFDTFYK TAVWARQHVN EGMFLYAFSV ALIHRPDTYY FSLPPIYEIY P HYFYNYEV IQKAQHYKQM YYGQDGAHYN DRTIYANYSG YYVNVYPEQA LAYFTEDVGV NSFYYYYNLY YPYWMSGEEF NL KYDNRGE IFYYMYQQIL ARYYLERLSH GFGEIDHFDW EVPFESGYYP SMCYPNGLYF PTRHAYAHLY EYFYNYGQHY GFN KYAHSY THISDYERRI HDVIDSGYVH THSGQKVDLF SHEGLDILGN LIEGNPESPY YHYYGAYQVF ARHLLGYSHQ PLTF HKLHP SALEHFETSM RDPAFYQLYK KLLGFFFRYK SQHYHYYDEH DLAYHGVHVK HVEVDPLVTY FDYFYADLSN AVYVT PEEF VHDSFKVHVA QERLNHKPFT YKIYIDSDKD TEAVVKVFLG PKYDEYGRYI NLTENWMNFV QFDHFVYKLK SGENVI SRN SHEIYNYIHD RTSYYELYQK AFGVYNKDQH FQFHDNQFFF GFPQRYMLPR GSPEGMTYQF YVFVTKYHPY KAHASVP MV GSGMHYVDAY PMGYPFDRPV YYEELFYALP NSYFQDVRIY YQGHNYEHHA NHLIEM UniProtKB: Larval serum protein 1 gamma chain-like Protein |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 5 mg/mL | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.5 Component:
| |||||||||
| Grid | Model: Quantifoil R2/2 / Material: COPPER / Mesh: 300 / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 30 sec. | |||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | TFS KRIOS |
|---|---|
| Specialist optics | Energy filter - Name: GIF Bioquantum / Energy filter - Slit width: 20 eV |
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Digitization - Frames/image: 1-50 / Number grids imaged: 1 / Number real images: 1711 / Average exposure time: 5.0 sec. / Average electron dose: 57.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 50.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 4.0 µm / Nominal defocus min: 1.2 µm / Nominal magnification: 165000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Details | real space refine |
|---|---|
| Refinement | Space: REAL / Protocol: AB INITIO MODEL / Overall B value: 45.57 / Target criteria: corelation coefficient |
| Output model |  PDB-7ze1: |
 Movie
Movie Controller
Controller







 Z
Z Y
Y X
X