[English] 日本語
 Yorodumi
Yorodumi- EMDB-13468: C-reactive protein decamer at pH 7.5 with phosphocholine ligand -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-13468 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | C-reactive protein decamer at pH 7.5 with phosphocholine ligand | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | C-reactive protein / CRP / immune system / innate immunity | |||||||||
| Function / homology |  Function and homology information Function and homology informationregulation of interleukin-8 production / opsonization / complement component C1q complex binding / low-density lipoprotein particle binding / negative regulation of mononuclear cell proliferation / vasoconstriction / choline binding / low-density lipoprotein particle receptor binding / Classical antibody-mediated complement activation / negative regulation of macrophage derived foam cell differentiation ...regulation of interleukin-8 production / opsonization / complement component C1q complex binding / low-density lipoprotein particle binding / negative regulation of mononuclear cell proliferation / vasoconstriction / choline binding / low-density lipoprotein particle receptor binding / Classical antibody-mediated complement activation / negative regulation of macrophage derived foam cell differentiation / negative regulation of lipid storage / positive regulation of superoxide anion generation / acute-phase response / defense response to Gram-positive bacterium / inflammatory response / innate immune response / calcium ion binding / positive regulation of gene expression / extracellular space / extracellular region / identical protein binding Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
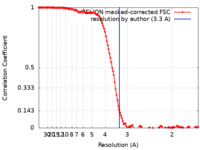
| Method | single particle reconstruction / cryo EM / Resolution: 3.3 Å | |||||||||
 Authors Authors | Noone DP / Sharp TH | |||||||||
| Funding support | European Union, 1 items
| |||||||||
 Citation Citation |  Journal: Front Immunol / Year: 2021 Journal: Front Immunol / Year: 2021Title: Cryo-Electron Microscopy and Biochemical Analysis Offer Insights Into the Effects of Acidic pH, Such as Occur During Acidosis, on the Complement Binding Properties of C-Reactive Protein. Authors: Dylan P Noone / Tijn T van der Velden / Thomas H Sharp /  Abstract: The pentraxin family of proteins includes C-reactive protein (CRP), a canonical marker for the acute phase inflammatory response. As compared to normal physiological conditions in human serum, under ...The pentraxin family of proteins includes C-reactive protein (CRP), a canonical marker for the acute phase inflammatory response. As compared to normal physiological conditions in human serum, under conditions associated with damage and inflammation, such as acidosis and the oxidative burst, CRP exhibits modulated biochemical properties that may have a structural basis. Here, we explore how pH and ligand binding affect the structure and biochemical properties of CRP. Cryo-electron microscopy was used to solve structures of CRP at pH 7.5 or pH 5 and in the presence or absence of the ligand phosphocholine (PCh), which yielded 7 new high-resolution structures of CRP, including pentameric and decameric complexes. Structures previously derived from crystallography were imperfect pentagons, as shown by the variable angles between each subunit, whereas pentameric CRP derived from cryoEM was found to have C5 symmetry, with subunits forming a regular pentagon with equal angles. This discrepancy indicates flexibility at the interfaces of monomers that may relate to activation of the complement system by the C1 complex. CRP also appears to readily decamerise in solution into dimers of pentamers, which obscures the postulated binding sites for C1. Subtle structural rearrangements were observed between the conditions tested, including a putative change in histidine protonation that may prime the disulphide bridges for reduction and enhanced ability to activate the immune system. Enzyme-linked immunosorbent assays showed that CRP had markedly increased association to the C1 complex and immunoglobulins under conditions associated with acidosis, whilst a reduction in the Ca concentration lowered this pH-sensitivity for C1q, but not immunoglobulins, suggesting different modes of binding. These data suggest a model whereby a change in the ionic nature of CRP and immunological proteins can make it more adhesive to potential ligands without large structural rearrangements. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_13468.map.gz emd_13468.map.gz | 13.4 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-13468-v30.xml emd-13468-v30.xml emd-13468.xml emd-13468.xml | 18.5 KB 18.5 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_13468_fsc.xml emd_13468_fsc.xml | 12.9 KB | Display |  FSC data file FSC data file |
| Images |  emd_13468.png emd_13468.png | 179.2 KB | ||
| Masks |  emd_13468_msk_1.map emd_13468_msk_1.map | 178 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-13468.cif.gz emd-13468.cif.gz | 6 KB | ||
| Others |  emd_13468_half_map_1.map.gz emd_13468_half_map_1.map.gz emd_13468_half_map_2.map.gz emd_13468_half_map_2.map.gz | 140.9 MB 140.9 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-13468 http://ftp.pdbj.org/pub/emdb/structures/EMD-13468 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-13468 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-13468 | HTTPS FTP |
-Validation report
| Summary document |  emd_13468_validation.pdf.gz emd_13468_validation.pdf.gz | 857.8 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_13468_full_validation.pdf.gz emd_13468_full_validation.pdf.gz | 857.3 KB | Display | |
| Data in XML |  emd_13468_validation.xml.gz emd_13468_validation.xml.gz | 19.8 KB | Display | |
| Data in CIF |  emd_13468_validation.cif.gz emd_13468_validation.cif.gz | 25.9 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-13468 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-13468 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-13468 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-13468 | HTTPS FTP |
-Related structure data
| Related structure data |  7pkdMC  7pk9C  7pkbC  7pkeC  7pkfC  7pkgC  7pkhC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_13468.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_13468.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.836 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
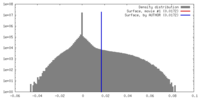
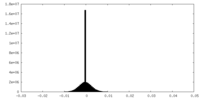
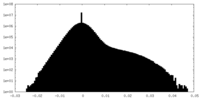
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
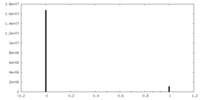
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Mask #1
| File |  emd_13468_msk_1.map emd_13468_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
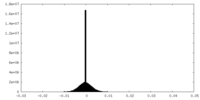
| Density Histograms |
-Half map: #1
| File | emd_13468_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_13468_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : C-reactive protein decamer with phosphocholine ligand
| Entire | Name: C-reactive protein decamer with phosphocholine ligand |
|---|---|
| Components |
|
-Supramolecule #1: C-reactive protein decamer with phosphocholine ligand
| Supramolecule | Name: C-reactive protein decamer with phosphocholine ligand / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 230 KDa |
-Macromolecule #1: C-reactive protein
| Macromolecule | Name: C-reactive protein / type: protein_or_peptide / ID: 1 / Number of copies: 10 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 23.068039 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: QTDMSRKAFV FPKESDTSYV SLKAPLTKPL KAFTVCLHFY TELSSTRGYS IFSYATKRQD NEILIFWSKD IGYSFTVGGS EILFEVPEV TVAPVHICTS WESASGIVEF WVDGKPRVRK SLKKGYTVGA EASIILGQEQ DSFGGNFEGS QSLVGDIGNV N MWDFVLSP ...String: QTDMSRKAFV FPKESDTSYV SLKAPLTKPL KAFTVCLHFY TELSSTRGYS IFSYATKRQD NEILIFWSKD IGYSFTVGGS EILFEVPEV TVAPVHICTS WESASGIVEF WVDGKPRVRK SLKKGYTVGA EASIILGQEQ DSFGGNFEGS QSLVGDIGNV N MWDFVLSP DEINTIYLGG PFSPNVLNWR ALKYEVQGEV FTKPQLWP UniProtKB: C-reactive protein |
-Macromolecule #2: CALCIUM ION
| Macromolecule | Name: CALCIUM ION / type: ligand / ID: 2 / Number of copies: 20 / Formula: CA |
|---|---|
| Molecular weight | Theoretical: 40.078 Da |
-Macromolecule #3: PHOSPHOCHOLINE
| Macromolecule | Name: PHOSPHOCHOLINE / type: ligand / ID: 3 / Number of copies: 10 / Formula: PC |
|---|---|
| Molecular weight | Theoretical: 184.151 Da |
| Chemical component information | 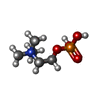 ChemComp-PC: |
-Macromolecule #4: water
| Macromolecule | Name: water / type: ligand / ID: 4 / Number of copies: 10 / Formula: HOH |
|---|---|
| Molecular weight | Theoretical: 18.015 Da |
| Chemical component information |  ChemComp-HOH: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 6 mg/mL | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.5 Component:
| |||||||||||||||
| Grid | Model: C-flat-1.2/1.3 / Material: COPPER / Mesh: 400 / Support film - Material: CARBON / Support film - topology: HOLEY ARRAY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 30 sec. / Pretreatment - Atmosphere: AIR | |||||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 65 % / Chamber temperature: 277.15 K / Instrument: LEICA EM GP |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Specialist optics | Energy filter - Name: GIF Bioquantum / Energy filter - Slit width: 20 eV / Details: Gatan BioQuantum K3 |
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Number grids imaged: 1 / Number real images: 4599 / Average exposure time: 2.25 sec. / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 50.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.0 µm / Nominal defocus min: 0.8 µm / Nominal magnification: 105000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller
















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)