+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-10397 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of yeast 80S ribosome stalled on poly(A) tract. | |||||||||
 Map data Map data | Yeast 80S ribosome stalled on a poly(A) tract | |||||||||
 Sample Sample |
| |||||||||
| Function / homology |  Function and homology information Function and homology informationmaturation of SSU-rRNA from tricistronic rRNA transcript (SSU-rRNA, LSU-rRNA,5S) / negative regulation of glucose mediated signaling pathway / negative regulation of translational frameshifting / RMTs methylate histone arginines / positive regulation of translational fidelity / Protein methylation / mTORC1-mediated signalling / Protein hydroxylation / ribosome-associated ubiquitin-dependent protein catabolic process / GDP-dissociation inhibitor activity ...maturation of SSU-rRNA from tricistronic rRNA transcript (SSU-rRNA, LSU-rRNA,5S) / negative regulation of glucose mediated signaling pathway / negative regulation of translational frameshifting / RMTs methylate histone arginines / positive regulation of translational fidelity / Protein methylation / mTORC1-mediated signalling / Protein hydroxylation / ribosome-associated ubiquitin-dependent protein catabolic process / GDP-dissociation inhibitor activity / hexon binding / positive regulation of nuclear-transcribed mRNA catabolic process, deadenylation-dependent decay / nonfunctional rRNA decay / pre-mRNA 5'-splice site binding / preribosome, small subunit precursor / Formation of the ternary complex, and subsequently, the 43S complex / Translation initiation complex formation / cleavage in ITS2 between 5.8S rRNA and LSU-rRNA of tricistronic rRNA transcript (SSU-rRNA, 5.8S rRNA, LSU-rRNA) / Ribosomal scanning and start codon recognition / response to cycloheximide / Major pathway of rRNA processing in the nucleolus and cytosol / mRNA destabilization / SRP-dependent cotranslational protein targeting to membrane / GTP hydrolysis and joining of the 60S ribosomal subunit / Nonsense Mediated Decay (NMD) independent of the Exon Junction Complex (EJC) / Nonsense Mediated Decay (NMD) enhanced by the Exon Junction Complex (EJC) / Formation of a pool of free 40S subunits / negative regulation of mRNA splicing, via spliceosome / preribosome, large subunit precursor / regulation of amino acid metabolic process / L13a-mediated translational silencing of Ceruloplasmin expression / translational elongation / ribosomal large subunit export from nucleus / 90S preribosome / G-protein alpha-subunit binding / positive regulation of protein kinase activity / endonucleolytic cleavage to generate mature 3'-end of SSU-rRNA from (SSU-rRNA, 5.8S rRNA, LSU-rRNA) / regulation of translational fidelity / protein-RNA complex assembly / ribosomal subunit export from nucleus / ribosomal small subunit export from nucleus / translation regulator activity / translational termination / endonucleolytic cleavage in ITS1 to separate SSU-rRNA from 5.8S rRNA and LSU-rRNA from tricistronic rRNA transcript (SSU-rRNA, 5.8S rRNA, LSU-rRNA) / DNA-(apurinic or apyrimidinic site) endonuclease activity / maturation of LSU-rRNA / cellular response to amino acid starvation / maturation of LSU-rRNA from tricistronic rRNA transcript (SSU-rRNA, 5.8S rRNA, LSU-rRNA) / ribosome assembly / rescue of stalled ribosome / ribosomal large subunit biogenesis / maturation of SSU-rRNA from tricistronic rRNA transcript (SSU-rRNA, 5.8S rRNA, LSU-rRNA) / maturation of SSU-rRNA / small-subunit processome / translational initiation / protein kinase C binding / macroautophagy / maintenance of translational fidelity / modification-dependent protein catabolic process / cytoplasmic stress granule / protein tag activity / rRNA processing / ribosomal small subunit biogenesis / small ribosomal subunit rRNA binding / ribosome biogenesis / viral capsid / ribosome binding / ribosomal small subunit assembly / small ribosomal subunit / 5S rRNA binding / large ribosomal subunit rRNA binding / cytosolic small ribosomal subunit / ribosomal large subunit assembly / cytoplasmic translation / cytosolic large ribosomal subunit / negative regulation of translation / rRNA binding / ribosome / protein ubiquitination / structural constituent of ribosome / positive regulation of protein phosphorylation / G protein-coupled receptor signaling pathway / translation / negative regulation of gene expression / response to antibiotic / mRNA binding / ubiquitin protein ligase binding / host cell nucleus / nucleolus / mitochondrion / RNA binding / zinc ion binding / nucleoplasm / nucleus / metal ion binding / cytosol / cytoplasm Similarity search - Function | |||||||||
| Biological species |   | |||||||||
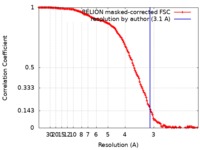
| Method | single particle reconstruction / cryo EM / Resolution: 3.1 Å | |||||||||
 Authors Authors | Tesina P / Buschauer R / Cheng J / Berninghausen O / Becker R / Beckmann R | |||||||||
| Funding support |  Germany, 1 items Germany, 1 items
| |||||||||
 Citation Citation |  Journal: EMBO J / Year: 2020 Journal: EMBO J / Year: 2020Title: Molecular mechanism of translational stalling by inhibitory codon combinations and poly(A) tracts. Authors: Petr Tesina / Laura N Lessen / Robert Buschauer / Jingdong Cheng / Colin Chih-Chien Wu / Otto Berninghausen / Allen R Buskirk / Thomas Becker / Roland Beckmann / Rachel Green /   Abstract: Inhibitory codon pairs and poly(A) tracts within the translated mRNA cause ribosome stalling and reduce protein output. The molecular mechanisms that drive these stalling events, however, are still ...Inhibitory codon pairs and poly(A) tracts within the translated mRNA cause ribosome stalling and reduce protein output. The molecular mechanisms that drive these stalling events, however, are still unknown. Here, we use a combination of in vitro biochemistry, ribosome profiling, and cryo-EM to define molecular mechanisms that lead to these ribosome stalls. First, we use an in vitro reconstituted yeast translation system to demonstrate that inhibitory codon pairs slow elongation rates which are partially rescued by increased tRNA concentration or by an artificial tRNA not dependent on wobble base-pairing. Ribosome profiling data extend these observations by revealing that paused ribosomes with empty A sites are enriched on these sequences. Cryo-EM structures of stalled ribosomes provide a structural explanation for the observed effects by showing decoding-incompatible conformations of mRNA in the A sites of all studied stall- and collision-inducing sequences. Interestingly, in the case of poly(A) tracts, the inhibitory conformation of the mRNA in the A site involves a nucleotide stacking array. Together, these data demonstrate a novel mRNA-induced mechanisms of translational stalling in eukaryotic ribosomes. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_10397.map.gz emd_10397.map.gz | 139.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-10397-v30.xml emd-10397-v30.xml emd-10397.xml emd-10397.xml | 96.8 KB 96.8 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_10397_fsc.xml emd_10397_fsc.xml | 14.2 KB | Display |  FSC data file FSC data file |
| Images |  emd_10397.png emd_10397.png | 230.1 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-10397 http://ftp.pdbj.org/pub/emdb/structures/EMD-10397 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-10397 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-10397 | HTTPS FTP |
-Validation report
| Summary document |  emd_10397_validation.pdf.gz emd_10397_validation.pdf.gz | 293.6 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_10397_full_validation.pdf.gz emd_10397_full_validation.pdf.gz | 292.8 KB | Display | |
| Data in XML |  emd_10397_validation.xml.gz emd_10397_validation.xml.gz | 13.9 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-10397 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-10397 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-10397 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-10397 | HTTPS FTP |
-Related structure data
| Related structure data |  6t7tMC  6t4qC  6t7iC  6t83C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_10397.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_10397.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Yeast 80S ribosome stalled on a poly(A) tract | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.084 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
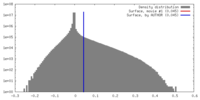
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
+Entire : Structure of yeast 80S ribosome stalled on poly(A) tract.
+Supramolecule #1: Structure of yeast 80S ribosome stalled on poly(A) tract.
+Supramolecule #2: yeast 80S ribosome
+Supramolecule #3: mRNA
+Macromolecule #1: 60S ribosomal protein L2-A
+Macromolecule #2: 40S ribosomal protein S0-A
+Macromolecule #3: 60S ribosomal protein L3
+Macromolecule #4: 40S ribosomal protein S1-A
+Macromolecule #6: 40S ribosomal protein S15
+Macromolecule #7: 40S ribosomal protein S2
+Macromolecule #8: 40S ribosomal protein S3
+Macromolecule #9: 40S ribosomal protein S4-A
+Macromolecule #10: Rps5p
+Macromolecule #11: 40S ribosomal protein S6-A
+Macromolecule #12: 40S ribosomal protein S7-A
+Macromolecule #13: 40S ribosomal protein S8
+Macromolecule #14: 40S ribosomal protein S9-A
+Macromolecule #15: 40S ribosomal protein S10-A
+Macromolecule #16: 40S ribosomal protein S11-A
+Macromolecule #17: 40S ribosomal protein S12
+Macromolecule #18: 40S ribosomal protein S13
+Macromolecule #19: 40S ribosomal protein S14-B
+Macromolecule #20: 40S ribosomal protein S16-A
+Macromolecule #21: 40S ribosomal protein S17-B
+Macromolecule #22: 40S ribosomal protein S18-A
+Macromolecule #23: 40S ribosomal protein S19-A
+Macromolecule #24: 40S ribosomal protein S20
+Macromolecule #25: 40S ribosomal protein S21-A
+Macromolecule #26: 40S ribosomal protein S22-A
+Macromolecule #27: 40S ribosomal protein S23-A
+Macromolecule #28: 40S ribosomal protein S24-A
+Macromolecule #29: 40S ribosomal protein S25-A
+Macromolecule #30: 40S ribosomal protein S26-B
+Macromolecule #31: 40S ribosomal protein S27-A
+Macromolecule #32: 40S ribosomal protein S29-A
+Macromolecule #33: 40S ribosomal protein S30-A
+Macromolecule #34: Ubiquitin-40S ribosomal protein S31
+Macromolecule #35: Guanine nucleotide-binding protein subunit beta-like protein
+Macromolecule #36: 40S ribosomal protein S28-A
+Macromolecule #39: 60S ribosomal protein L4-A
+Macromolecule #40: 60S ribosomal protein L5
+Macromolecule #41: 60S ribosomal protein L6-B
+Macromolecule #42: 60S ribosomal protein L7-A
+Macromolecule #43: 60S ribosomal protein L8-A
+Macromolecule #44: 60S ribosomal protein L9-A
+Macromolecule #45: 60S ribosomal protein L10
+Macromolecule #46: 60S ribosomal protein L11-B
+Macromolecule #47: 60S ribosomal protein L13-A
+Macromolecule #48: 60S ribosomal protein L14-A
+Macromolecule #49: 60S ribosomal protein L15-A
+Macromolecule #50: 60S ribosomal protein L16-A
+Macromolecule #51: 60S ribosomal protein L17-A
+Macromolecule #52: 60S ribosomal protein L18-A
+Macromolecule #53: 60S ribosomal protein L19-A
+Macromolecule #54: 60S ribosomal protein L20-A
+Macromolecule #55: 60S ribosomal protein L21-A
+Macromolecule #56: 60S ribosomal protein L22-A
+Macromolecule #57: 60S ribosomal protein L23-A
+Macromolecule #58: 60S ribosomal protein L24-A
+Macromolecule #59: 60S ribosomal protein L25
+Macromolecule #60: 60S ribosomal protein L26-A
+Macromolecule #61: 60S ribosomal protein L27-A
+Macromolecule #62: 60S ribosomal protein L28
+Macromolecule #63: 60S ribosomal protein L29
+Macromolecule #64: 60S ribosomal protein L30
+Macromolecule #65: 60S ribosomal protein L31-A
+Macromolecule #66: 60S ribosomal protein L32
+Macromolecule #67: 60S ribosomal protein L33-A
+Macromolecule #68: 60S ribosomal protein L34-A
+Macromolecule #69: 60S ribosomal protein L35-A
+Macromolecule #70: 60S ribosomal protein L36-A
+Macromolecule #71: 60S ribosomal protein L37-A
+Macromolecule #72: 60S ribosomal protein L38
+Macromolecule #73: 60S ribosomal protein L39
+Macromolecule #74: Ubiquitin-60S ribosomal protein L40
+Macromolecule #75: 60S ribosomal protein L41-A
+Macromolecule #76: 60S ribosomal protein L42-A
+Macromolecule #77: 60S ribosomal protein L43-A
+Macromolecule #81: nascent chain
+Macromolecule #5: 18S rRNA
+Macromolecule #37: 5S rRNA
+Macromolecule #38: 5.8S rRNA
+Macromolecule #78: 25S rRNA
+Macromolecule #79: mRNA
+Macromolecule #80: tRNA
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.2 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON II (4k x 4k) / Average electron dose: 2.5 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller


























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)