[English] 日本語
 Yorodumi
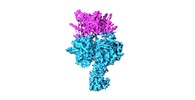
Yorodumi- EMDB-10213: Structure of the human DDB1-DDA1-DCAF15 E3 ubiquitin ligase bound... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-10213 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of the human DDB1-DDA1-DCAF15 E3 ubiquitin ligase bound to RBM39 and Indisulam | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
| Function / homology |  Function and homology information Function and homology informationRS domain binding / regulation of natural killer cell activation / positive regulation by virus of viral protein levels in host cell / epigenetic programming in the zygotic pronuclei / spindle assembly involved in female meiosis / U1 snRNP binding / Cul4-RING E3 ubiquitin ligase complex / UV-damage excision repair / regulation of mRNA splicing, via spliceosome / biological process involved in interaction with symbiont ...RS domain binding / regulation of natural killer cell activation / positive regulation by virus of viral protein levels in host cell / epigenetic programming in the zygotic pronuclei / spindle assembly involved in female meiosis / U1 snRNP binding / Cul4-RING E3 ubiquitin ligase complex / UV-damage excision repair / regulation of mRNA splicing, via spliceosome / biological process involved in interaction with symbiont / regulation of mitotic cell cycle phase transition / Cul4A-RING E3 ubiquitin ligase complex / WD40-repeat domain binding / Cul4B-RING E3 ubiquitin ligase complex / ubiquitin ligase complex scaffold activity / immune system process / negative regulation of reproductive process / negative regulation of developmental process / cullin family protein binding / viral release from host cell / centriolar satellite / small molecule binding / ectopic germ cell programmed cell death / proteasomal protein catabolic process / positive regulation of viral genome replication / RNA processing / positive regulation of gluconeogenesis / mRNA Splicing - Major Pathway / RNA splicing / nucleotide-excision repair / Recognition of DNA damage by PCNA-containing replication complex / DNA Damage Recognition in GG-NER / regulation of circadian rhythm / Dual Incision in GG-NER / Transcription-Coupled Nucleotide Excision Repair (TC-NER) / Formation of TC-NER Pre-Incision Complex / mRNA processing / Wnt signaling pathway / Formation of Incision Complex in GG-NER / Dual incision in TC-NER / Gap-filling DNA repair synthesis and ligation in TC-NER / protein polyubiquitination / positive regulation of protein catabolic process / cellular response to UV / microtubule cytoskeleton / positive regulation of proteasomal ubiquitin-dependent protein catabolic process / rhythmic process / protein-macromolecule adaptor activity / Neddylation / site of double-strand break / ubiquitin-dependent protein catabolic process / proteasome-mediated ubiquitin-dependent protein catabolic process / chromosome, telomeric region / damaged DNA binding / protein ubiquitination / nuclear speck / DNA repair / apoptotic process / DNA damage response / protein-containing complex binding / nucleolus / negative regulation of apoptotic process / protein-containing complex / DNA binding / RNA binding / extracellular space / extracellular exosome / nucleoplasm / nucleus / metal ion binding / cytoplasm Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
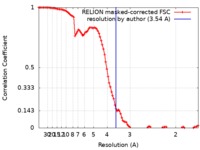
| Method | single particle reconstruction / cryo EM / Resolution: 3.54 Å | |||||||||
 Authors Authors | Srinivas H | |||||||||
 Citation Citation |  Journal: Nat Chem Biol / Year: 2020 Journal: Nat Chem Biol / Year: 2020Title: Structural basis of indisulam-mediated RBM39 recruitment to DCAF15 E3 ligase complex. Authors: Dirksen E Bussiere / Lili Xie / Honnappa Srinivas / Wei Shu / Ashley Burke / Celine Be / Junping Zhao / Adarsh Godbole / Dan King / Rajeshri G Karki / Viktor Hornak / Fangmin Xu / Jennifer ...Authors: Dirksen E Bussiere / Lili Xie / Honnappa Srinivas / Wei Shu / Ashley Burke / Celine Be / Junping Zhao / Adarsh Godbole / Dan King / Rajeshri G Karki / Viktor Hornak / Fangmin Xu / Jennifer Cobb / Nathalie Carte / Andreas O Frank / Alexandra Frommlet / Patrick Graff / Mark Knapp / Aleem Fazal / Barun Okram / Songchun Jiang / Pierre-Yves Michellys / Rohan Beckwith / Hans Voshol / Christian Wiesmann / Jonathan M Solomon / Joshiawa Paulk /   Abstract: The anticancer agent indisulam inhibits cell proliferation by causing degradation of RBM39, an essential mRNA splicing factor. Indisulam promotes an interaction between RBM39 and the DCAF15 E3 ligase ...The anticancer agent indisulam inhibits cell proliferation by causing degradation of RBM39, an essential mRNA splicing factor. Indisulam promotes an interaction between RBM39 and the DCAF15 E3 ligase substrate receptor, leading to RBM39 ubiquitination and proteasome-mediated degradation. To delineate the precise mechanism by which indisulam mediates the DCAF15-RBM39 interaction, we solved the DCAF15-DDB1-DDA1-indisulam-RBM39(RRM2) complex structure to a resolution of 2.3 Å. DCAF15 has a distinct topology that embraces the RBM39(RRM2) domain largely via non-polar interactions, and indisulam binds between DCAF15 and RBM39(RRM2), coordinating additional interactions between the two proteins. Studies with RBM39 point mutants and indisulam analogs validated the structural model and defined the RBM39 α-helical degron motif. The degron is found only in RBM23 and RBM39, and only these proteins were detectably downregulated in indisulam-treated HCT116 cells. This work further explains how indisulam induces RBM39 degradation and defines the challenge of harnessing DCAF15 to degrade additional targets. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_10213.map.gz emd_10213.map.gz | 5.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-10213-v30.xml emd-10213-v30.xml emd-10213.xml emd-10213.xml | 16.4 KB 16.4 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_10213_fsc.xml emd_10213_fsc.xml | 10 KB | Display |  FSC data file FSC data file |
| Images |  emd_10213.png emd_10213.png | 115.3 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-10213 http://ftp.pdbj.org/pub/emdb/structures/EMD-10213 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-10213 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-10213 | HTTPS FTP |
-Validation report
| Summary document |  emd_10213_validation.pdf.gz emd_10213_validation.pdf.gz | 242 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_10213_full_validation.pdf.gz emd_10213_full_validation.pdf.gz | 241.1 KB | Display | |
| Data in XML |  emd_10213_validation.xml.gz emd_10213_validation.xml.gz | 11.3 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-10213 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-10213 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-10213 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-10213 | HTTPS FTP |
-Related structure data
| Related structure data |  6sj7MC  6ud7C  6ue5C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_10213.map.gz / Format: CCP4 / Size: 83.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_10213.map.gz / Format: CCP4 / Size: 83.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Voxel size | X=Y=Z: 0.86 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : DDB1 complex
| Entire | Name: DDB1 complex |
|---|---|
| Components |
|
-Supramolecule #1: DDB1 complex
| Supramolecule | Name: DDB1 complex / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#4 |
|---|---|
| Molecular weight | Experimental: 210 KDa |
-Supramolecule #2: DDB1-DDA1-DCAF15-RBM39 complex
| Supramolecule | Name: DDB1-DDA1-DCAF15-RBM39 complex / type: complex / ID: 2 / Parent: 1 / Macromolecule list: #1-#4 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Recombinant expression | Organism:  |
-Supramolecule #3: RBM39
| Supramolecule | Name: RBM39 / type: complex / ID: 3 / Parent: 1 / Macromolecule list: #3 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Recombinant expression | Organism:  |
-Macromolecule #1: DDB1- and CUL4-associated factor 15
| Macromolecule | Name: DDB1- and CUL4-associated factor 15 / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 66.563297 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: GPAPSSKSER NSGAGSGGGG PGGAGGKRAA GRRREHVLKQ LERVKISGQL SPRLFRKLPP RVCVSLKNIV DEDFLYAGHI FLGFSKCGR YVLSYTSSSG DDDFSFYIYH LYWWEFNVHS KLKLVRQVRL FQDEEIYSDL YLTVCEWPSD ASKVIVFGFN T RSANGMLM ...String: GPAPSSKSER NSGAGSGGGG PGGAGGKRAA GRRREHVLKQ LERVKISGQL SPRLFRKLPP RVCVSLKNIV DEDFLYAGHI FLGFSKCGR YVLSYTSSSG DDDFSFYIYH LYWWEFNVHS KLKLVRQVRL FQDEEIYSDL YLTVCEWPSD ASKVIVFGFN T RSANGMLM NMMMMSDENH RDIYVSTVAV PPPGRCAACQ DASRAHPGDP NAQCLRHGFM LHTKYQVVYP FPTFQPAFQL KK DQVVLLN TSYSLVACAV SVHSAGDRSF CQILYDHSTC PLAPASPPEP QSPELPPALP SFCPEAAPAR SSGSPEPSPA IAK AKEFVA DIFRRAKEAK GGVPEEARPA LCPGPSGSRC RAHSEPLALC GETAPRDSPP ASEAPASEPG YVNYTKLYYV LESG EGTEP EDELEDDKIS LPFVVTDLRG RNLRPMRERT AVQGQYLTVE QLTLDFEYVI NEVIRHDATW GHQFCSFSDY DIVIL EVCP ETNQVLINIG LLLLAFPSPT EEGQLRPKTY HTSLKVAWDL NTGIFETVSV GDLTEVKGQT SGSVWSSYRK SCVDMV MKW LVPESSGRYV NRMTNEALHK GCSLKVLADS ERYTWIVL |
-Macromolecule #2: DNA damage-binding protein 1
| Macromolecule | Name: DNA damage-binding protein 1 / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 127.097469 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MSYNYVVTAQ KPTAVNGCVT GHFTSAEDLN LLIAKNTRLE IYVVTAEGLR PVKEVGMYGK IAVMELFRPK GESKDLLFIL TAKYNACIL EYKQSGESID IITRAHGNVQ DRIGRPSETG IIGIIDPECR MIGLRLYDGL FKVIPLDRDN KELKAFNIRL E ELHVIDVK ...String: MSYNYVVTAQ KPTAVNGCVT GHFTSAEDLN LLIAKNTRLE IYVVTAEGLR PVKEVGMYGK IAVMELFRPK GESKDLLFIL TAKYNACIL EYKQSGESID IITRAHGNVQ DRIGRPSETG IIGIIDPECR MIGLRLYDGL FKVIPLDRDN KELKAFNIRL E ELHVIDVK FLYGCQAPTI CFVYQDPQGR HVKTYEVSLR EKEFNKGPWK QENVEAEASM VIAVPEPFGG AIIIGQESIT YH NGDKYLA IAPPIIKQST IVCHNRVDPN GSRYLLGDME GRLFMLLLEK EEQMDGTVTL KDLRVELLGE TSIAECLTYL DNG VVFVGS RLGDSQLVKL NVDSNEQGSY VVAMETFTNL GPIVDMCVVD LERQGQGQLV TCSGAFKEGS LRIIRNGIGI HEHA SIDLP GIKGLWPLRS DPNRETDDTL VLSFVGQTRV LMLNGEEVEE TELMGFVDDQ QTFFCGNVAH QQLIQITSAS VRLVS QEPK ALVSEWKEPQ AKNISVASCN SSQVVVAVGR ALYYLQIHPQ ELRQISHTEM EHEVACLDIT PLGDSNGLSP LCAIGL WTD ISARILKLPS FELLHKEMLG GEIIPRSILM TTFESSHYLL CALGDGALFY FGLNIETGLL SDRKKVTLGT QPTVLRT FR SLSTTNVFAC SDRPTVIYSS NHKLVFSNVN LKEVNYMCPL NSDGYPDSLA LANNSTLTIG TIDEIQKLHI RTVPLYES P RKICYQEVSQ CFGVLSSRIE VQDTSGGTTA LRPSASTQAL SSSVSSSKLF SSSTAPHETS FGEEVEVHNL LIIDQHTFE VLHAHQFLQN EYALSLVSCK LGKDPNTYFI VGTAMVYPEE AEPKQGRIVV FQYSDGKLQT VAEKEVKGAV YSMVEFNGKL LASINSTVR LYEWTTEKEL RTECNHYNNI MALYLKTKGD FILVGDLMRS VLLLAYKPME GNFEEIARDF NPNWMSAVEI L DDDNFLGA ENAFNLFVCQ KDSAATTDEE RQHLQEVGLF HLGEFVNVFC HGSLVMQNLG ETSTPTQGSV LFGTVNGMIG LV TSLSESW YNLLLDMQNR LNKVIKSVGK IEHSFWRSFH TERKTEPATG FIDGDLIESF LDISRPKMQE VVANLQYDDG SGM KREATA DDLIKVVEEL TRIH |
-Macromolecule #3: RNA binding protein 39
| Macromolecule | Name: RNA binding protein 39 / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 9.085409 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: GPMRLYVGSL HFNITEDMLR GIFEPFGRIE SIQLMMDSET GRSKGYGFIT FSDSECAKKA LEQLNGFELA GRPMKVGHVT E |
-Macromolecule #4: DET1- and DDB1-associated protein 1
| Macromolecule | Name: DET1- and DDB1-associated protein 1 / type: protein_or_peptide / ID: 4 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 11.724102 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: ADFLKGLPVY NKSNFSRFHA DSVCKASNRR PSVYLPTREY PSEQIIVTEK TNILLRYLHQ QWDKKNAAKK RDQEQVELEG ESSAPPRKV ARTDSPDMHE DT |
-Macromolecule #5: N~1~-(3-chloro-1H-indol-7-yl)benzene-1,4-disulfonamide
| Macromolecule | Name: N~1~-(3-chloro-1H-indol-7-yl)benzene-1,4-disulfonamide type: ligand / ID: 5 / Number of copies: 1 / Formula: EF6 |
|---|---|
| Molecular weight | Theoretical: 385.846 Da |
| Chemical component information | 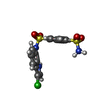 ChemComp-EF6: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 QUANTUM (4k x 4k) / Detector mode: COUNTING / Average electron dose: 40.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller