+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 6q0r | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of DDB1-DDA1-DCAF15 complex bound to E7820 and RBM39 | |||||||||
 Components Components |
| |||||||||
 Keywords Keywords | LIGASE / Ubiquitin / homeostasis / targeted protein degradation | |||||||||
| Function / homology |  Function and homology information Function and homology informationRS domain binding / regulation of natural killer cell activation / U1 snRNP binding / regulation of mRNA splicing, via spliceosome / positive regulation by virus of viral protein levels in host cell / spindle assembly involved in female meiosis / epigenetic programming in the zygotic pronuclei / UV-damage excision repair / biological process involved in interaction with symbiont / WD40-repeat domain binding ...RS domain binding / regulation of natural killer cell activation / U1 snRNP binding / regulation of mRNA splicing, via spliceosome / positive regulation by virus of viral protein levels in host cell / spindle assembly involved in female meiosis / epigenetic programming in the zygotic pronuclei / UV-damage excision repair / biological process involved in interaction with symbiont / WD40-repeat domain binding / regulation of mitotic cell cycle phase transition / Cul4A-RING E3 ubiquitin ligase complex / Cul4-RING E3 ubiquitin ligase complex / small molecule binding / Cul4B-RING E3 ubiquitin ligase complex / ubiquitin ligase complex scaffold activity / negative regulation of reproductive process / negative regulation of developmental process / viral release from host cell / cullin family protein binding / immune system process / ectopic germ cell programmed cell death / RNA processing / positive regulation of viral genome replication / proteasomal protein catabolic process / sperm end piece / mRNA Splicing - Major Pathway / positive regulation of gluconeogenesis / sperm principal piece / RNA splicing / nucleotide-excision repair / Recognition of DNA damage by PCNA-containing replication complex / regulation of circadian rhythm / DNA Damage Recognition in GG-NER / centriolar satellite / Dual Incision in GG-NER / Transcription-Coupled Nucleotide Excision Repair (TC-NER) / Formation of TC-NER Pre-Incision Complex / Wnt signaling pathway / mRNA processing / Formation of Incision Complex in GG-NER / protein polyubiquitination / Dual incision in TC-NER / Gap-filling DNA repair synthesis and ligation in TC-NER / positive regulation of protein catabolic process / cellular response to UV / sperm midpiece / rhythmic process / positive regulation of proteasomal ubiquitin-dependent protein catabolic process / site of double-strand break / microtubule cytoskeleton / Neddylation / protein-macromolecule adaptor activity / ubiquitin-dependent protein catabolic process / proteasome-mediated ubiquitin-dependent protein catabolic process / damaged DNA binding / chromosome, telomeric region / nuclear speck / protein ubiquitination / DNA repair / apoptotic process / DNA damage response / negative regulation of apoptotic process / protein-containing complex binding / nucleolus / protein-containing complex / extracellular space / DNA binding / RNA binding / extracellular exosome / nucleoplasm / metal ion binding / nucleus / cytoplasm Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.9 Å MOLECULAR REPLACEMENT / Resolution: 2.9 Å | |||||||||
 Authors Authors | Faust, T. / Yoon, H. / Nowak, R.P. / Donovan, K.A. / Li, Z. / Cai, Q. / Eleuteri, N.A. / Zhang, T. / Gray, N.S. / Fischer, E.S. | |||||||||
| Funding support |  United States, 2items United States, 2items
| |||||||||
 Citation Citation |  Journal: Nat Chem Biol / Year: 2020 Journal: Nat Chem Biol / Year: 2020Title: Structural complementarity facilitates E7820-mediated degradation of RBM39 by DCAF15. Authors: Tyler B Faust / Hojong Yoon / Radosław P Nowak / Katherine A Donovan / Zhengnian Li / Quan Cai / Nicholas A Eleuteri / Tinghu Zhang / Nathanael S Gray / Eric S Fischer /  Abstract: The investigational drugs E7820, indisulam and tasisulam (aryl-sulfonamides) promote the degradation of the splicing factor RBM39 in a proteasome-dependent mechanism. While the activity critically ...The investigational drugs E7820, indisulam and tasisulam (aryl-sulfonamides) promote the degradation of the splicing factor RBM39 in a proteasome-dependent mechanism. While the activity critically depends on the cullin RING ligase substrate receptor DCAF15, the molecular details remain elusive. Here we present the cryo-EM structure of the DDB1-DCAF15-DDA1 core ligase complex bound to RBM39 and E7820 at a resolution of 4.4 Å, together with crystal structures of engineered subcomplexes. We show that DCAF15 adopts a new fold stabilized by DDA1, and that extensive protein-protein contacts between the ligase and substrate mitigate low affinity interactions between aryl-sulfonamides and DCAF15. Our data demonstrate how aryl-sulfonamides neo-functionalize a shallow, non-conserved pocket on DCAF15 to selectively bind and degrade RBM39 and the closely related splicing factor RBM23 without the requirement for a high-affinity ligand, which has broad implications for the de novo discovery of molecular glue degraders. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  6q0r.cif.gz 6q0r.cif.gz | 549.6 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb6q0r.ent.gz pdb6q0r.ent.gz | 447.5 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  6q0r.json.gz 6q0r.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/q0/6q0r https://data.pdbj.org/pub/pdb/validation_reports/q0/6q0r ftp://data.pdbj.org/pub/pdb/validation_reports/q0/6q0r ftp://data.pdbj.org/pub/pdb/validation_reports/q0/6q0r | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  6q0vC  6q0wC  5fqdS S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
-Protein , 3 types, 3 molecules ADE
| #1: Protein | Mass: 96425.586 Da / Num. of mol.: 1 / Fragment: internal deletion of the BPB domain Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: DDB1, XAP1 / Production host: Homo sapiens (human) / Gene: DDB1, XAP1 / Production host:  Trichoplusia ni (cabbage looper) / References: UniProt: Q16531 Trichoplusia ni (cabbage looper) / References: UniProt: Q16531 |
|---|---|
| #4: Protein | Mass: 12062.565 Da / Num. of mol.: 1 / Fragment: RRM2 domain Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: RBM39, HCC1, RNPC2 / Production host: Homo sapiens (human) / Gene: RBM39, HCC1, RNPC2 / Production host:  Trichoplusia ni (cabbage looper) / References: UniProt: Q14498 Trichoplusia ni (cabbage looper) / References: UniProt: Q14498 |
| #5: Protein | Mass: 14542.154 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: DDA1, C19orf58, PCIA1 / Production host: Homo sapiens (human) / Gene: DDA1, C19orf58, PCIA1 / Production host:  Trichoplusia ni (cabbage looper) / References: UniProt: Q9BW61 Trichoplusia ni (cabbage looper) / References: UniProt: Q9BW61 |
-DDB1- and CUL4-associated factor ... , 2 types, 2 molecules BC
| #2: Protein | Mass: 31611.043 Da / Num. of mol.: 1 / Fragment: N-terminal domain Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: DCAF15, C19orf72 / Production host: Homo sapiens (human) / Gene: DCAF15, C19orf72 / Production host:  Trichoplusia ni (cabbage looper) / References: UniProt: Q66K64 Trichoplusia ni (cabbage looper) / References: UniProt: Q66K64 |
|---|---|
| #3: Protein | Mass: 29893.537 Da / Num. of mol.: 1 / Fragment: C-terminal domain Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: DCAF15, C19orf72 / Production host: Homo sapiens (human) / Gene: DCAF15, C19orf72 / Production host:  Trichoplusia ni (cabbage looper) / References: UniProt: Q66K64 Trichoplusia ni (cabbage looper) / References: UniProt: Q66K64 |
-Non-polymers , 4 types, 19 molecules 
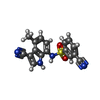





| #6: Chemical | ChemComp-OXM / |
|---|---|
| #7: Chemical | ChemComp-O6M / |
| #8: Chemical | ChemComp-ZN / |
| #9: Water | ChemComp-HOH / |
-Details
| Has ligand of interest | Y |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.65 Å3/Da / Density % sol: 53.62 % |
|---|---|
| Crystal grow | Temperature: 300 K / Method: vapor diffusion / Details: 20% PEG 4000 |
-Data collection
| Diffraction | Mean temperature: 100 K / Ambient temp details: N2 / Serial crystal experiment: N |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  APS APS  / Beamline: 24-ID-C / Wavelength: 0.9792 Å / Beamline: 24-ID-C / Wavelength: 0.9792 Å |
| Detector | Type: DECTRIS PILATUS 6M-F / Detector: PIXEL / Date: Feb 8, 2019 |
| Radiation | Monochromator: Cryo cooled double crystal / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.9792 Å / Relative weight: 1 |
| Reflection | Resolution: 2.9→50 Å / Num. obs: 44491 / % possible obs: 99.5 % / Redundancy: 6.7 % / Biso Wilson estimate: 102.82 Å2 / CC1/2: 1 / Rmerge(I) obs: 0.03 / Net I/σ(I): 14.7 |
| Reflection shell | Resolution: 2.9→3 Å / Rmerge(I) obs: 0.8 / Num. unique obs: 4381 / CC1/2: 0.69 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 5fqd chain A Resolution: 2.9→46.05 Å / Cor.coef. Fo:Fc: 0.933 / Cor.coef. Fo:Fc free: 0.9 / SU R Cruickshank DPI: 1.016 / Cross valid method: THROUGHOUT / SU R Blow DPI: 0.936 / SU Rfree Blow DPI: 0.341 / SU Rfree Cruickshank DPI: 0.349
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 133.23 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine analyze | Luzzati coordinate error obs: 0.44 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.9→46.05 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 2.9→2.92 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Refine-ID: X-RAY DIFFRACTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group |
|
 Movie
Movie Controller
Controller















 PDBj
PDBj









