[English] 日本語
 Yorodumi
Yorodumi- EMDB-0856: Cryo-EM structure of echovirus 11 complexed with its attaching re... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-0856 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of echovirus 11 complexed with its attaching receptor CD55 at pH 7.4 | |||||||||
 Map data Map data | Cryo-EM structure of echovirus 11 complexed with its attaching receptor CD55 at pH 7.4 | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Cryo-EM structure / echovirus 11 / CD55 / pH 7.4 / VIRUS | |||||||||
| Function / homology |  Function and homology information Function and homology informationregulation of lipopolysaccharide-mediated signaling pathway / negative regulation of complement activation / regulation of complement-dependent cytotoxicity / regulation of complement activation / respiratory burst / positive regulation of CD4-positive, alpha-beta T cell activation / positive regulation of CD4-positive, alpha-beta T cell proliferation / Class B/2 (Secretin family receptors) / symbiont-mediated suppression of host cytoplasmic pattern recognition receptor signaling pathway via inhibition of RIG-I activity / ficolin-1-rich granule membrane ...regulation of lipopolysaccharide-mediated signaling pathway / negative regulation of complement activation / regulation of complement-dependent cytotoxicity / regulation of complement activation / respiratory burst / positive regulation of CD4-positive, alpha-beta T cell activation / positive regulation of CD4-positive, alpha-beta T cell proliferation / Class B/2 (Secretin family receptors) / symbiont-mediated suppression of host cytoplasmic pattern recognition receptor signaling pathway via inhibition of RIG-I activity / ficolin-1-rich granule membrane / complement activation, classical pathway / COPI-mediated anterograde transport / side of membrane / transport vesicle / endoplasmic reticulum-Golgi intermediate compartment membrane / secretory granule membrane / Regulation of Complement cascade / picornain 2A / symbiont-mediated suppression of host mRNA export from nucleus / symbiont genome entry into host cell via pore formation in plasma membrane / picornain 3C / T=pseudo3 icosahedral viral capsid / positive regulation of T cell cytokine production / host cell cytoplasmic vesicle membrane / viral capsid / host cell / nucleoside-triphosphate phosphatase / virus receptor activity / channel activity / positive regulation of cytosolic calcium ion concentration / monoatomic ion transmembrane transport / DNA replication / RNA helicase activity / membrane raft / endocytosis involved in viral entry into host cell / symbiont-mediated activation of host autophagy / symbiont entry into host cell / Golgi membrane / RNA-directed RNA polymerase / cysteine-type endopeptidase activity / viral RNA genome replication / innate immune response / symbiont-mediated suppression of host gene expression / RNA-directed RNA polymerase activity / DNA-templated transcription / : / lipid binding / Neutrophil degranulation / host cell nucleus / virion attachment to host cell / structural molecule activity / cell surface / ATP hydrolysis activity / proteolysis / RNA binding / extracellular exosome / extracellular region / ATP binding / membrane / metal ion binding / plasma membrane Similarity search - Function | |||||||||
| Biological species |   Echovirus E11 / Echovirus E11 /  Homo sapiens (human) Homo sapiens (human) | |||||||||
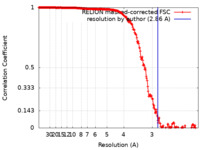
| Method | single particle reconstruction / cryo EM / Resolution: 2.86 Å | |||||||||
 Authors Authors | Liu S / Gao FG | |||||||||
 Citation Citation |  Journal: Chin.Sci.Bull. / Year: 2020 Journal: Chin.Sci.Bull. / Year: 2020Title: Molecular and structural basis of Echovirus 11 infection by using the dual-receptor system of CD55 and FcRn. Authors: Niu S / Liu C / Liu C / Liu S / Song Y / Zhang Y / Tian W / Zhao X / Wang P / Gao FG | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_0856.map.gz emd_0856.map.gz | 202.6 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-0856-v30.xml emd-0856-v30.xml emd-0856.xml emd-0856.xml | 12.7 KB 12.7 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_0856_fsc.xml emd_0856_fsc.xml | 22.5 KB | Display |  FSC data file FSC data file |
| Images |  emd_0856.png emd_0856.png | 263.9 KB | ||
| Filedesc metadata |  emd-0856.cif.gz emd-0856.cif.gz | 5.6 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-0856 http://ftp.pdbj.org/pub/emdb/structures/EMD-0856 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-0856 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-0856 | HTTPS FTP |
-Validation report
| Summary document |  emd_0856_validation.pdf.gz emd_0856_validation.pdf.gz | 658.5 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_0856_full_validation.pdf.gz emd_0856_full_validation.pdf.gz | 658 KB | Display | |
| Data in XML |  emd_0856_validation.xml.gz emd_0856_validation.xml.gz | 16.9 KB | Display | |
| Data in CIF |  emd_0856_validation.cif.gz emd_0856_validation.cif.gz | 24.2 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-0856 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-0856 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-0856 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-0856 | HTTPS FTP |
-Related structure data
| Related structure data |  6la5MC  0854C  0855C  0857C  0858C  0859C  0860C  0867C  0870C  0871C  6la3C  6la4C  6la6C  6la7C  6laoC  6lapC  6lb1C  6lboC  6lbqC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_0856.map.gz / Format: CCP4 / Size: 216 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_0856.map.gz / Format: CCP4 / Size: 216 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Cryo-EM structure of echovirus 11 complexed with its attaching receptor CD55 at pH 7.4 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.09 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
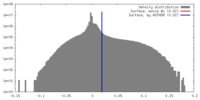
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Echovirus E11
| Entire | Name:   Echovirus E11 Echovirus E11 |
|---|---|
| Components |
|
-Supramolecule #1: Echovirus E11
| Supramolecule | Name: Echovirus E11 / type: virus / ID: 1 / Parent: 0 / Macromolecule list: #2-#5 / NCBI-ID: 12078 / Sci species name: Echovirus E11 / Virus type: VIRION / Virus isolate: STRAIN / Virus enveloped: No / Virus empty: No |
|---|
-Macromolecule #1: Capsid protein VP1
| Macromolecule | Name: Capsid protein VP1 / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Echovirus E11 Echovirus E11 |
| Molecular weight | Theoretical: 32.277359 KDa |
| Sequence | String: VVEAVENAVA RVADTISSGP SNSQAVPALT AVETGHTSQV TPSDTIQTRH VRNYHSRSES SIENFLCRSA CVYMGEYHTT NTDTSKLFA SWTINARRMV QMRRKLELFT YVRFDMEVTF VITSKQDQGT QLGQDMPPLT HQIMYIPPGG PIPKSVTDYT W QTSTNPSI ...String: VVEAVENAVA RVADTISSGP SNSQAVPALT AVETGHTSQV TPSDTIQTRH VRNYHSRSES SIENFLCRSA CVYMGEYHTT NTDTSKLFA SWTINARRMV QMRRKLELFT YVRFDMEVTF VITSKQDQGT QLGQDMPPLT HQIMYIPPGG PIPKSVTDYT W QTSTNPSI FWTEGNAPPR MSIPFISIGN AYSNFYDGWS HFSQNGVYGY NTLNHMGQIY VRHVNGSSPL PMTSTVRMYF KP KHVKVWV PRPPRLCQYK NASTVNFTPT NITEKRQSIN YIPETVKP |
-Macromolecule #2: Capsid protein VP2
| Macromolecule | Name: Capsid protein VP2 / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Echovirus E11 Echovirus E11 |
| Molecular weight | Theoretical: 27.968449 KDa |
| Sequence | String: DRVRSITLGN STITTQESAN VVVAYGRWPE YLKDNEATAE DQPTQPDVAT CRFYTLESVT WERDSPGWWW KFPDALKDMG LFGQNMYYH YLGRAGYTIH VQCNASKFHQ GCLMVVCVPE AEMGCSQVDG TVNEHSLSEG ETAKKFASTS TNGTNTVQSI V TNAGMGVG ...String: DRVRSITLGN STITTQESAN VVVAYGRWPE YLKDNEATAE DQPTQPDVAT CRFYTLESVT WERDSPGWWW KFPDALKDMG LFGQNMYYH YLGRAGYTIH VQCNASKFHQ GCLMVVCVPE AEMGCSQVDG TVNEHSLSEG ETAKKFASTS TNGTNTVQSI V TNAGMGVG VGNLTIFPHQ WINLRTNNCA TIVMPYINNV PMDNMFRHHN FTLMIIPFVP LDYSSDSSTY VPITVTVAPM CA EYNGLRL ATSL |
-Macromolecule #3: Capsid protein VP3
| Macromolecule | Name: Capsid protein VP3 / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Echovirus E11 Echovirus E11 |
| Molecular weight | Theoretical: 26.062578 KDa |
| Sequence | String: GLPVMNTPGS NQFLTSDDFQ SPSAMPQFDV TPELNIPGEV QNLMEIAEVD SVVPVNNVEG KLDTMEIYRI PVQSGNHQSS QVFGFQVQP GLDNVFKHTL LGEILNYYAH WSGSIKLTFV FCGSAMATGK FLLAYAPPGA NAPKSRKDAM LGTHIIWDVG L QSSCVLCI ...String: GLPVMNTPGS NQFLTSDDFQ SPSAMPQFDV TPELNIPGEV QNLMEIAEVD SVVPVNNVEG KLDTMEIYRI PVQSGNHQSS QVFGFQVQP GLDNVFKHTL LGEILNYYAH WSGSIKLTFV FCGSAMATGK FLLAYAPPGA NAPKSRKDAM LGTHIIWDVG L QSSCVLCI PWISQTHYRL VQQDEYTSAG NVTCWYQTGI VVPAGTPTSC SIMCFVSACN DFSVRLLKDT PFIEQSALLQ |
-Macromolecule #4: Capsid protein VP4
| Macromolecule | Name: Capsid protein VP4 / type: protein_or_peptide / ID: 4 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Echovirus E11 Echovirus E11 |
| Molecular weight | Theoretical: 7.495273 KDa |
| Sequence | String: MGAQVSTQKT GAHETGLNAS GRSIIHYTNI NYYKDAASNS ANRQDFSQDP GKFTEPVKDI MVKSLPALN |
-Macromolecule #5: Complement decay-accelerating factor
| Macromolecule | Name: Complement decay-accelerating factor / type: protein_or_peptide / ID: 5 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 13.586049 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: KSCPNPGEIR NGQIDVPGGI LFGATISFSC NTGYKLFGST SSFCLISGSS VQWSDPLPEC REIYCPAPPQ IDNGIIQGER DHYGYRQSV TYACNKGFTM IGEHSIYCTV NNDEGEWSGP PPECRG UniProtKB: Complement decay-accelerating factor |
-Macromolecule #6: SPHINGOSINE
| Macromolecule | Name: SPHINGOSINE / type: ligand / ID: 6 / Number of copies: 1 / Formula: SPH |
|---|---|
| Molecular weight | Theoretical: 299.492 Da |
| Chemical component information |  ChemComp-SPH: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON III (4k x 4k) / Average electron dose: 1.025 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller



















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)