[English] 日本語
 Yorodumi
Yorodumi- PDB-6yx8: The structure of allophycocyanin from cyanobacterium Nostoc sp. W... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 6yx8 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | The structure of allophycocyanin from cyanobacterium Nostoc sp. WR13, the C2221 crystal form. | ||||||||||||
 Components Components |
| ||||||||||||
 Keywords Keywords |  PHOTOSYNTHESIS / Allophycocyanin crystal structure Phycobilisome Phycobiliproteins Phycocyanobilin chromophore Light harvesting complex Cyanobacterium Nostoc sp. WR13 PHOTOSYNTHESIS / Allophycocyanin crystal structure Phycobilisome Phycobiliproteins Phycocyanobilin chromophore Light harvesting complex Cyanobacterium Nostoc sp. WR13 | ||||||||||||
| Function / homology |  Function and homology information Function and homology information phycobilisome / plasma membrane-derived thylakoid membrane / phycobilisome / plasma membrane-derived thylakoid membrane /  photosynthesis photosynthesisSimilarity search - Function | ||||||||||||
| Biological species |  Nostoc sp. WR13 (bacteria) Nostoc sp. WR13 (bacteria) | ||||||||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.831 Å MOLECULAR REPLACEMENT / Resolution: 1.831 Å | ||||||||||||
 Authors Authors | Patel, H.M. / Roszak, A.W. / Madamwar, D. / Cogdell, R.J. | ||||||||||||
| Funding support |  United States, United States,  India, 3items India, 3items
| ||||||||||||
 Citation Citation |  Journal: To Be Published Journal: To Be PublishedTitle: The high resolution structure of allophycocyanin from cyanobacterium Nostoc sp. WR13 Authors: Patel, H.M. / Roszak, A.W. / Madamwar, D. / Cogdell, R.J. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  6yx8.cif.gz 6yx8.cif.gz | 405.3 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb6yx8.ent.gz pdb6yx8.ent.gz | Display |  PDB format PDB format | |
| PDBx/mmJSON format |  6yx8.json.gz 6yx8.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/yx/6yx8 https://data.pdbj.org/pub/pdb/validation_reports/yx/6yx8 ftp://data.pdbj.org/pub/pdb/validation_reports/yx/6yx8 ftp://data.pdbj.org/pub/pdb/validation_reports/yx/6yx8 | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  6yx7SC S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| |||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
| |||||||||||||||||||||||||||||||||||
| Unit cell |
| |||||||||||||||||||||||||||||||||||
| Components on special symmetry positions |
| |||||||||||||||||||||||||||||||||||
| Noncrystallographic symmetry (NCS) | NCS domain:
NCS ensembles :
|
- Components
Components
-Protein , 2 types, 6 molecules AAACCCEEEBBBDDDFFF
| #1: Protein | Mass: 17007.270 Da / Num. of mol.: 3 / Source method: isolated from a natural source Details: THERE IS ONE CHROMOPHORE MOLECULE, PHYCOCYANOBILIN (LIGAND ID: CYC), BOUND COVALENTLY TO ALLOPHYCOCYANIN ALPHA SUBUNIT RESIDUE CYS80; The N-terminal Methionine present in the gene sequence ...Details: THERE IS ONE CHROMOPHORE MOLECULE, PHYCOCYANOBILIN (LIGAND ID: CYC), BOUND COVALENTLY TO ALLOPHYCOCYANIN ALPHA SUBUNIT RESIDUE CYS80; The N-terminal Methionine present in the gene sequence was post-translationally removed and is not present in the crystal structure Source: (natural)  Nostoc sp. WR13 (bacteria) Nostoc sp. WR13 (bacteria)Plasmid details: From the desert Rann of Kachchh (RoK), Gujarat, India References: UniProt: A0A4Y5PW22 #2: Protein | Mass: 17340.707 Da / Num. of mol.: 3 / Source method: isolated from a natural source Details: THERE IS ONE CHROMOPHORE MOLECULE, PHYCOCYANOBILIN (LIGAND ID: CYC), BOUND COVALENTLY TO ALLOPHYCOCYANIN BETA SUBUNIT RESIDUE CYS81 Source: (natural)  Nostoc sp. WR13 (bacteria) Nostoc sp. WR13 (bacteria)Plasmid details: From the desert Rann of Kachchh (RoK), Gujarat, India References: UniProt: A0A4Y5PW23 |
|---|
-Non-polymers , 10 types, 1027 molecules 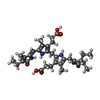

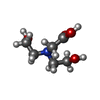
















| #3: Chemical | ChemComp-PXQ / #4: Chemical | ChemComp-MPD / (  2-Methyl-2,4-pentanediol 2-Methyl-2,4-pentanediol#5: Chemical |  Bicine Bicine#6: Chemical | ChemComp-1PE /  Polyethylene glycol Polyethylene glycol#7: Chemical | ChemComp-PGE /  Polyethylene glycol Polyethylene glycol#8: Chemical | ChemComp-PEG /  Diethylene glycol Diethylene glycol#9: Chemical | ChemComp-EDO /  Ethylene glycol Ethylene glycol#10: Chemical | ChemComp-PG4 /  Polyethylene glycol Polyethylene glycol#11: Chemical | ChemComp-MRD / ( |  2-Methyl-2,4-pentanediol 2-Methyl-2,4-pentanediol#12: Water | ChemComp-HOH / |  Water Water |
|---|
-Details
| Has ligand of interest | Y |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.66 Å3/Da / Density % sol: 53.7 % / Description: Blue color plates with orthorhombic morphology |
|---|---|
Crystal grow | Temperature: 293 K / Method: vapor diffusion, sitting drop / pH: 8.5 Details: Morpheus screen condition E12: 37.5% v/v Precipitant mix 4: 25% v/v MPD; 25% PEG 1000; 25% w/v PEG 3350; 0.1M Buffer system 3: 1.0M Tris (base); bicine, pH 8.5 0.12M Additives: 0.3M ethylene glycols |
-Data collection
| Diffraction | Mean temperature: 100 K / Serial crystal experiment: N |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  Diamond Diamond  / Beamline: I04-1 / Wavelength: 0.91587 Å / Beamline: I04-1 / Wavelength: 0.91587 Å |
| Detector | Type: DECTRIS PILATUS 6M-F / Detector: PIXEL / Date: Sep 7, 2018 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength : 0.91587 Å / Relative weight: 1 : 0.91587 Å / Relative weight: 1 |
| Reflection | Resolution: 1.831→90.144 Å / Num. obs: 87249 / % possible obs: 95.7 % / Redundancy: 6.6 % / Biso Wilson estimate: 26.15 Å2 / CC1/2: 0.996 / Rmerge(I) obs: 0.126 / Rpim(I) all: 0.053 / Rrim(I) all: 0.137 / Net I/σ(I): 9.3 |
| Reflection shell | Resolution: 1.831→1.941 Å / Rmerge(I) obs: 1.278 / Mean I/σ(I) obs: 1.5 / Num. unique obs: 4360 / CC1/2: 0.599 / Rpim(I) all: 0.524 / Rrim(I) all: 1.278 / % possible all: 58.3 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure : :  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 6YX7 Resolution: 1.831→90.144 Å / Cor.coef. Fo:Fc: 0.967 / Cor.coef. Fo:Fc free: 0.952 / SU B: 7.48 / SU ML: 0.096 / Cross valid method: FREE R-VALUE / ESU R: 0.142 / ESU R Free: 0.13 Details: Hydrogens have been added in their riding positions
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.2 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 32.791 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.831→90.144 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller












 PDBj
PDBj







