[English] 日本語
 Yorodumi
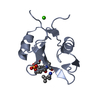
Yorodumi- PDB-3in7: Crystal Structure of the Grb2 SH2 Domain in Complex with a Cyclop... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 3in7 | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal Structure of the Grb2 SH2 Domain in Complex with a Cyclopropyl-constrained Ac-pY-Q-N-NH2 Tripeptide Mimic | ||||||
 Components Components | Growth factor receptor-bound protein 2 GRB2 GRB2 | ||||||
 Keywords Keywords |  SIGNALING PROTEIN/PEPTIDE / LIGAND PREORGANIZATION / PEPTIDE MIMICS / SIGNALING PROTEIN/PEPTIDE / LIGAND PREORGANIZATION / PEPTIDE MIMICS /  Golgi apparatus / Host-virus interaction / Golgi apparatus / Host-virus interaction /  Phosphoprotein / Phosphoprotein /  SH2 domain / SH2 domain /  SH3 domain / SH3 domain /  Signaling protein-pseudopeptide ligand complex / Signaling protein-pseudopeptide ligand complex /  SIGNALING PROTEIN-PEPTIDE COMPLEX SIGNALING PROTEIN-PEPTIDE COMPLEX | ||||||
| Function / homology |  Function and homology information Function and homology informationguanyl-nucleotide exchange factor adaptor activity / Grb2-EGFR complex / branching involved in labyrinthine layer morphogenesis / STAT5 Activation /  COP9 signalosome / vesicle membrane / neurotrophin TRKA receptor binding / Activated NTRK2 signals through PI3K / MET receptor recycling / COP9 signalosome / vesicle membrane / neurotrophin TRKA receptor binding / Activated NTRK2 signals through PI3K / MET receptor recycling /  transmembrane receptor protein tyrosine kinase adaptor activity ...guanyl-nucleotide exchange factor adaptor activity / Grb2-EGFR complex / branching involved in labyrinthine layer morphogenesis / STAT5 Activation / transmembrane receptor protein tyrosine kinase adaptor activity ...guanyl-nucleotide exchange factor adaptor activity / Grb2-EGFR complex / branching involved in labyrinthine layer morphogenesis / STAT5 Activation /  COP9 signalosome / vesicle membrane / neurotrophin TRKA receptor binding / Activated NTRK2 signals through PI3K / MET receptor recycling / COP9 signalosome / vesicle membrane / neurotrophin TRKA receptor binding / Activated NTRK2 signals through PI3K / MET receptor recycling /  transmembrane receptor protein tyrosine kinase adaptor activity / Signaling by cytosolic FGFR1 fusion mutants / Interleukin-15 signaling / MET activates PTPN11 / MET activates RAP1 and RAC1 / CD28 dependent Vav1 pathway / Costimulation by the CD28 family / MET activates PI3K/AKT signaling / Signal regulatory protein family interactions / Regulation of KIT signaling / transmembrane receptor protein tyrosine kinase adaptor activity / Signaling by cytosolic FGFR1 fusion mutants / Interleukin-15 signaling / MET activates PTPN11 / MET activates RAP1 and RAC1 / CD28 dependent Vav1 pathway / Costimulation by the CD28 family / MET activates PI3K/AKT signaling / Signal regulatory protein family interactions / Regulation of KIT signaling /  epidermal growth factor receptor binding / positive regulation of actin filament polymerization / PI-3K cascade:FGFR3 / STAT5 activation downstream of FLT3 ITD mutants / PI-3K cascade:FGFR2 / PI-3K cascade:FGFR4 / PI-3K cascade:FGFR1 / endodermal cell differentiation / epidermal growth factor receptor binding / positive regulation of actin filament polymerization / PI-3K cascade:FGFR3 / STAT5 activation downstream of FLT3 ITD mutants / PI-3K cascade:FGFR2 / PI-3K cascade:FGFR4 / PI-3K cascade:FGFR1 / endodermal cell differentiation /  regulation of MAPK cascade / GRB2:SOS provides linkage to MAPK signaling for Integrins / RHOU GTPase cycle / PI3K events in ERBB2 signaling / Signaling by ALK fusions and activated point mutants / SOS-mediated signalling / Activated NTRK3 signals through RAS / RET signaling / Activated NTRK2 signals through RAS / regulation of MAPK cascade / GRB2:SOS provides linkage to MAPK signaling for Integrins / RHOU GTPase cycle / PI3K events in ERBB2 signaling / Signaling by ALK fusions and activated point mutants / SOS-mediated signalling / Activated NTRK3 signals through RAS / RET signaling / Activated NTRK2 signals through RAS /  insulin receptor substrate binding / PI3K Cascade / insulin receptor substrate binding / PI3K Cascade /  Interleukin-3, Interleukin-5 and GM-CSF signaling / SHC1 events in ERBB4 signaling / RHO GTPases Activate WASPs and WAVEs / Signalling to RAS / fibroblast growth factor receptor signaling pathway / GAB1 signalosome / SHC-related events triggered by IGF1R / Activated NTRK2 signals through FRS2 and FRS3 / Role of LAT2/NTAL/LAB on calcium mobilization / Signal attenuation / Interleukin receptor SHC signaling / SHC-mediated cascade:FGFR3 / MET activates RAS signaling / Schwann cell development / Signaling by PDGFRA transmembrane, juxtamembrane and kinase domain mutants / Signaling by PDGFRA extracellular domain mutants / SHC-mediated cascade:FGFR2 / SHC-mediated cascade:FGFR4 / Signaling by FGFR4 in disease / SHC-mediated cascade:FGFR1 / Erythropoietin activates RAS / Interleukin-3, Interleukin-5 and GM-CSF signaling / SHC1 events in ERBB4 signaling / RHO GTPases Activate WASPs and WAVEs / Signalling to RAS / fibroblast growth factor receptor signaling pathway / GAB1 signalosome / SHC-related events triggered by IGF1R / Activated NTRK2 signals through FRS2 and FRS3 / Role of LAT2/NTAL/LAB on calcium mobilization / Signal attenuation / Interleukin receptor SHC signaling / SHC-mediated cascade:FGFR3 / MET activates RAS signaling / Schwann cell development / Signaling by PDGFRA transmembrane, juxtamembrane and kinase domain mutants / Signaling by PDGFRA extracellular domain mutants / SHC-mediated cascade:FGFR2 / SHC-mediated cascade:FGFR4 / Signaling by FGFR4 in disease / SHC-mediated cascade:FGFR1 / Erythropoietin activates RAS /  signal transduction in response to DNA damage / FRS-mediated FGFR3 signaling / Signaling by CSF3 (G-CSF) / Signaling by FLT3 ITD and TKD mutants / FRS-mediated FGFR2 signaling / FRS-mediated FGFR4 signaling / Signaling by FGFR3 in disease / FRS-mediated FGFR1 signaling / Tie2 Signaling / Signaling by FGFR2 in disease / GRB2 events in EGFR signaling / SHC1 events in EGFR signaling / signal transduction in response to DNA damage / FRS-mediated FGFR3 signaling / Signaling by CSF3 (G-CSF) / Signaling by FLT3 ITD and TKD mutants / FRS-mediated FGFR2 signaling / FRS-mediated FGFR4 signaling / Signaling by FGFR3 in disease / FRS-mediated FGFR1 signaling / Tie2 Signaling / Signaling by FGFR2 in disease / GRB2 events in EGFR signaling / SHC1 events in EGFR signaling /  myelination / EGFR Transactivation by Gastrin / Signaling by FLT3 fusion proteins / FLT3 Signaling / Signaling by FGFR1 in disease / GRB2 events in ERBB2 signaling / NCAM signaling for neurite out-growth / phosphotyrosine residue binding / myelination / EGFR Transactivation by Gastrin / Signaling by FLT3 fusion proteins / FLT3 Signaling / Signaling by FGFR1 in disease / GRB2 events in ERBB2 signaling / NCAM signaling for neurite out-growth / phosphotyrosine residue binding /  ephrin receptor binding / SHC1 events in ERBB2 signaling / Downstream signal transduction / Insulin receptor signalling cascade / Constitutive Signaling by Overexpressed ERBB2 / FCERI mediated Ca+2 mobilization / InlB-mediated entry of Listeria monocytogenes into host cell / insulin-like growth factor receptor signaling pathway / Signaling by phosphorylated juxtamembrane, extracellular and kinase domain KIT mutants / Antigen activates B Cell Receptor (BCR) leading to generation of second messengers / cellular response to ionizing radiation / Regulation of signaling by CBL / Negative regulation of FGFR3 signaling / Negative regulation of FGFR2 signaling / FCGR3A-mediated phagocytosis / FCERI mediated MAPK activation / Negative regulation of FGFR4 signaling / EGFR downregulation / Negative regulation of FGFR1 signaling / Signaling by ERBB2 TMD/JMD mutants / B cell receptor signaling pathway ephrin receptor binding / SHC1 events in ERBB2 signaling / Downstream signal transduction / Insulin receptor signalling cascade / Constitutive Signaling by Overexpressed ERBB2 / FCERI mediated Ca+2 mobilization / InlB-mediated entry of Listeria monocytogenes into host cell / insulin-like growth factor receptor signaling pathway / Signaling by phosphorylated juxtamembrane, extracellular and kinase domain KIT mutants / Antigen activates B Cell Receptor (BCR) leading to generation of second messengers / cellular response to ionizing radiation / Regulation of signaling by CBL / Negative regulation of FGFR3 signaling / Negative regulation of FGFR2 signaling / FCGR3A-mediated phagocytosis / FCERI mediated MAPK activation / Negative regulation of FGFR4 signaling / EGFR downregulation / Negative regulation of FGFR1 signaling / Signaling by ERBB2 TMD/JMD mutants / B cell receptor signaling pathwaySimilarity search - Function | ||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  MOLECULAR REPLACEMENT / MOLECULAR REPLACEMENT /  molecular replacement / Resolution: 2 Å molecular replacement / Resolution: 2 Å | ||||||
 Authors Authors | Clements, J.H. | ||||||
 Citation Citation |  Journal: J.Am.Chem.Soc. / Year: 2009 Journal: J.Am.Chem.Soc. / Year: 2009Title: Thermodynamic and Structural Effects of Conformational Constraints in Protein-Ligand Interactions. Entropic Paradoxy Associated with Ligand Preorganization. Authors: Delorbe, J.E. / Clements, J.H. / Teresk, M.G. / Benfield, A.P. / Plake, H.R. / Millspaugh, L.E. / Martin, S.F. #1:  Journal: Angew.Chem.Int.Ed.Engl. / Year: 2006 Journal: Angew.Chem.Int.Ed.Engl. / Year: 2006Title: Ligand Preorganization May Be Accompanied by Entropic Penalties in Protein-Ligand Interactions Authors: Benfield, A.P. / Teresk, M.G. / Plake, H.R. / DeLorbe, J.E. / Millspaugh, L.E. / Martin, S.F. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  3in7.cif.gz 3in7.cif.gz | 59.8 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb3in7.ent.gz pdb3in7.ent.gz | 42.5 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  3in7.json.gz 3in7.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/in/3in7 https://data.pdbj.org/pub/pdb/validation_reports/in/3in7 ftp://data.pdbj.org/pub/pdb/validation_reports/in/3in7 ftp://data.pdbj.org/pub/pdb/validation_reports/in/3in7 | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  3imdC  3imjC  3in8C  3kfjC  2huwS S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| 2 | 
| ||||||||
| 3 |
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein |  GRB2 / Adapter protein GRB2 / SH2/SH3 adapter GRB2 / Protein Ash GRB2 / Adapter protein GRB2 / SH2/SH3 adapter GRB2 / Protein AshMass: 13758.543 Da / Num. of mol.: 2 / Fragment: SH2 domain Source method: isolated from a genetically manipulated source Details: residues 53-163 were expressed in addition to a C-terminal 6-his tag Source: (gene. exp.)   Homo sapiens (human) / Gene: GRB2, ASH / Plasmid: pQE-60 / Production host: Homo sapiens (human) / Gene: GRB2, ASH / Plasmid: pQE-60 / Production host:   Escherichia coli (E. coli) / Strain (production host): SG13009 / References: UniProt: P62993 Escherichia coli (E. coli) / Strain (production host): SG13009 / References: UniProt: P62993#2: Chemical | #3: Water | ChemComp-HOH / |  Water Water |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.18 Å3/Da / Density % sol: 43.47 % |
|---|---|
Crystal grow | Temperature: 298 K / Method: vapor diffusion, hanging drop / pH: 7.5 Details: Ligand in lyophilized powder form was dissolved in a 7.6 mg/mL solution of Grb2 SH2 in water such to give a protein/ligand molar ratio of 1.7:1. 3.5 uL of this solution was mixed with 3.5 uL ...Details: Ligand in lyophilized powder form was dissolved in a 7.6 mg/mL solution of Grb2 SH2 in water such to give a protein/ligand molar ratio of 1.7:1. 3.5 uL of this solution was mixed with 3.5 uL of 0.1 M HEPES, 20% w/v PEG MW10,000, pH 7.5 to create the hanging drop, which yielded crystals of the protein-ligand complex in the presence of the above-mentioned solution after two weeks at room temperature., VAPOR DIFFUSION, HANGING DROP, temperature 298K |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  ROTATING ANODE / Type: RIGAKU RU200 / Wavelength: 1.5418 Å ROTATING ANODE / Type: RIGAKU RU200 / Wavelength: 1.5418 Å |
| Detector | Type: RIGAKU RAXIS IV++ / Detector: IMAGE PLATE / Date: May 25, 2007 |
| Radiation | Monochromator: blue max-flux confocal / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength : 1.5418 Å / Relative weight: 1 : 1.5418 Å / Relative weight: 1 |
| Reflection | Resolution: 1.7→50 Å / Num. all: 27887 / Num. obs: 19967 / % possible obs: 71.6 % / Observed criterion σ(F): 0 / Observed criterion σ(I): 0 / Redundancy: 4.5 % / Rmerge(I) obs: 0.054 / Χ2: 1.75 / Net I/σ(I): 20.4 |
| Reflection shell | Resolution: 1.7→1.76 Å / Redundancy: 1.7 % / Rmerge(I) obs: 0.305 / Num. unique all: 179 / Χ2: 0.989 / % possible all: 6.6 |
-Phasing
Phasing | Method:  molecular replacement molecular replacement |
|---|
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure : :  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: pdb entry 2HUW Resolution: 2→50 Å / Occupancy max: 1 / Occupancy min: 1 / Cross valid method: THROUGHOUT / σ(F): 0 / σ(I): 0 / Stereochemistry target values: Engh & Huber
| ||||||||||||||||||||||||||||||||||||
| Solvent computation | Bsol: 20.711 Å2 | ||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso max: 64.88 Å2 / Biso mean: 31.558 Å2 / Biso min: 14.71 Å2
| ||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2→50 Å
| ||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||
| Xplor file |
|
 Movie
Movie Controller
Controller











 PDBj
PDBj


























