+ Open data
Open data
- Basic information
Basic information
| Entry | Database: SASBDB / ID: SASDEH2 |
|---|---|
 Sample Sample | Unlabeled nucleoporin NUP49/NSP49 (N49) without denaturant
|
| Function / homology |  Function and homology information Function and homology informationnuclear pore central transport channel / telomere tethering at nuclear periphery / nuclear pore cytoplasmic filaments / post-transcriptional tethering of RNA polymerase II gene DNA at nuclear periphery / tRNA export from nucleus / structural constituent of nuclear pore / RNA export from nucleus / nuclear localization sequence binding / nucleocytoplasmic transport / poly(A)+ mRNA export from nucleus ...nuclear pore central transport channel / telomere tethering at nuclear periphery / nuclear pore cytoplasmic filaments / post-transcriptional tethering of RNA polymerase II gene DNA at nuclear periphery / tRNA export from nucleus / structural constituent of nuclear pore / RNA export from nucleus / nuclear localization sequence binding / nucleocytoplasmic transport / poly(A)+ mRNA export from nucleus / ribosomal large subunit export from nucleus / nuclear pore / molecular condensate scaffold activity / protein import into nucleus / nuclear envelope / nuclear membrane / amyloid fibril formation / RNA binding / identical protein binding Similarity search - Function |
| Biological species |  |
 Citation Citation |  Journal: Proc Natl Acad Sci U S A / Year: 2017 Journal: Proc Natl Acad Sci U S A / Year: 2017Title: Decoupling of size and shape fluctuations in heteropolymeric sequences reconciles discrepancies in SAXS vs. FRET measurements. Authors: Gustavo Fuertes / Niccolò Banterle / Kiersten M Ruff / Aritra Chowdhury / Davide Mercadante / Christine Koehler / Michael Kachala / Gemma Estrada Girona / Sigrid Milles / Ankur Mishra / ...Authors: Gustavo Fuertes / Niccolò Banterle / Kiersten M Ruff / Aritra Chowdhury / Davide Mercadante / Christine Koehler / Michael Kachala / Gemma Estrada Girona / Sigrid Milles / Ankur Mishra / Patrick R Onck / Frauke Gräter / Santiago Esteban-Martín / Rohit V Pappu / Dmitri I Svergun / Edward A Lemke /     Abstract: Unfolded states of proteins and native states of intrinsically disordered proteins (IDPs) populate heterogeneous conformational ensembles in solution. The average sizes of these heterogeneous ...Unfolded states of proteins and native states of intrinsically disordered proteins (IDPs) populate heterogeneous conformational ensembles in solution. The average sizes of these heterogeneous systems, quantified by the radius of gyration ( ), can be measured by small-angle X-ray scattering (SAXS). Another parameter, the mean dye-to-dye distance ( ) for proteins with fluorescently labeled termini, can be estimated using single-molecule Förster resonance energy transfer (smFRET). A number of studies have reported inconsistencies in inferences drawn from the two sets of measurements for the dimensions of unfolded proteins and IDPs in the absence of chemical denaturants. These differences are typically attributed to the influence of fluorescent labels used in smFRET and to the impact of high concentrations and averaging features of SAXS. By measuring the dimensions of a collection of labeled and unlabeled polypeptides using smFRET and SAXS, we directly assessed the contributions of dyes to the experimental values and For chemically denatured proteins we obtain mutual consistency in our inferences based on and , whereas for IDPs under native conditions, we find substantial deviations. Using computations, we show that discrepant inferences are neither due to methodological shortcomings of specific measurements nor due to artifacts of dyes. Instead, our analysis suggests that chemical heterogeneity in heteropolymeric systems leads to a decoupling between and that is amplified in the absence of denaturants. Therefore, joint assessments of and combined with measurements of polymer shapes should provide a consistent and complete picture of the underlying ensembles. |
 Contact author Contact author |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
-Data source
| SASBDB page |  SASDEH2 SASDEH2 |
|---|
-Related structure data
| Related structure data | C: citing same article ( |
|---|---|
| Similar structure data |
- External links
External links
| Related items in Molecule of the Month |
|---|
-Models
| Model #2246 |  Type: atomic / Software: (CAMPARI) Comment: p-acetylphenylalanine was replaced with a cysteine. Chi-square value: 0.956 / P-value: 0.184774  Search similar-shape structures of this assembly by Omokage search (details) Search similar-shape structures of this assembly by Omokage search (details) |
|---|---|
| Model #2247 |  Type: atomic / Software: (CAMPARI) Comment: p-acetylphenylalanine was replaced with a cysteine. Chi-square value: 0.956 / P-value: 0.184774  Search similar-shape structures of this assembly by Omokage search (details) Search similar-shape structures of this assembly by Omokage search (details) |
| Model #2248 |  Type: atomic / Software: (CAMPARI) Comment: p-acetylphenylalanine was replaced with a cysteine. Chi-square value: 0.956 / P-value: 0.184774  Search similar-shape structures of this assembly by Omokage search (details) Search similar-shape structures of this assembly by Omokage search (details) |
| Model #2249 |  Type: atomic / Software: (CAMPARI) Comment: p-acetylphenylalanine was replaced with a cysteine. Chi-square value: 0.956 / P-value: 0.184774  Search similar-shape structures of this assembly by Omokage search (details) Search similar-shape structures of this assembly by Omokage search (details) |
| Model #2250 |  Type: atomic / Software: (CAMPARI) Comment: p-acetylphenylalanine was replaced with a cysteine. Chi-square value: 0.956 / P-value: 0.184774  Search similar-shape structures of this assembly by Omokage search (details) Search similar-shape structures of this assembly by Omokage search (details) |
| Model #2251 |  Type: atomic / Software: (CAMPARI) Comment: p-acetylphenylalanine was replaced with a cysteine. Chi-square value: 0.956 / P-value: 0.184774  Search similar-shape structures of this assembly by Omokage search (details) Search similar-shape structures of this assembly by Omokage search (details) |
| Model #2252 |  Type: atomic / Software: (CAMPARI) Comment: p-acetylphenylalanine was replaced with a cysteine. Chi-square value: 0.956 / P-value: 0.184774  Search similar-shape structures of this assembly by Omokage search (details) Search similar-shape structures of this assembly by Omokage search (details) |
| Model #2253 |  Type: atomic / Software: (CAMPARI) Comment: p-acetylphenylalanine was replaced with a cysteine. Chi-square value: 0.956 / P-value: 0.184774  Search similar-shape structures of this assembly by Omokage search (details) Search similar-shape structures of this assembly by Omokage search (details) |
| Model #2254 |  Type: atomic / Software: (CAMPARI) Comment: p-acetylphenylalanine was replaced with a cysteine. Chi-square value: 0.956 / P-value: 0.184774  Search similar-shape structures of this assembly by Omokage search (details) Search similar-shape structures of this assembly by Omokage search (details) |
- Sample
Sample
 Sample Sample | Name: Unlabeled nucleoporin NUP49/NSP49 (N49) without denaturant Specimen concentration: 2.00-10.00 |
|---|---|
| Buffer | Name: PBS, 10 mM DTT / pH: 7.4 |
| Entity #1229 | Name: N49 / Type: protein / Description: Nucleoporin NUP49/NSP49 / Formula weight: 3.853 / Num. of mol.: 1 Source: Saccharomyces cerevisiae (strain ATCC 204508 / S288c) References: UniProt: Q02199 Sequence: GCQTSRGLFG NNNTNNINNS SSGMNNASAG LFGSKPUA |
-Experimental information
| Beam | Instrument name: PETRA III EMBL P12 / City: Hamburg / 国: Germany  / Type of source: X-ray synchrotron / Wavelength: 0.1 Å / Dist. spec. to detc.: 3 mm / Type of source: X-ray synchrotron / Wavelength: 0.1 Å / Dist. spec. to detc.: 3 mm | |||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Detector | Name: Pilatus 2M | |||||||||||||||||||||||||||||||||
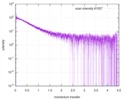
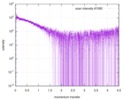
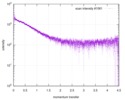
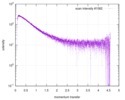
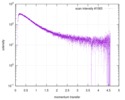
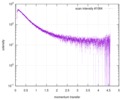
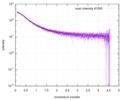
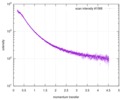
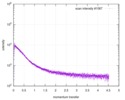
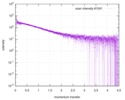
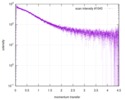
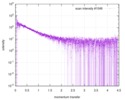
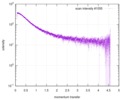
| Scan | Measurement date: Dec 8, 2013 / Cell temperature: 23 °C / Exposure time: 0.05 sec. / Number of frames: 20 / Unit: 1/nm /
| |||||||||||||||||||||||||||||||||
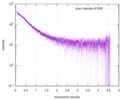
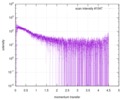
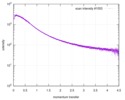
| Distance distribution function P(R) |
| |||||||||||||||||||||||||||||||||
| Result | Comments: The protein contains a penultimate non-canonical amino acid p-acetylphenylalanine (207 Da) that is represented as U (selenocysteine, 168 Da) in the amino acid sequence for the entry. ...Comments: The protein contains a penultimate non-canonical amino acid p-acetylphenylalanine (207 Da) that is represented as U (selenocysteine, 168 Da) in the amino acid sequence for the entry. Therefore, the calculated MW from sequence (MW(expected)) must be adjusted accordingly (ca. 40 Da).
|
 Movie
Movie Controller
Controller