+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 9j03 | ||||||
|---|---|---|---|---|---|---|---|
| Title | Cyro-EM Structure of Human TLR4/MD-2/DLAM1 Complex | ||||||
 Components Components |
| ||||||
 Keywords Keywords | IMMUNE SYSTEM / Innate immune system / Toll-like receptors / TLR4 agonist / Vaccine adjuvants / Disaccharide-based Lipid A Mimetics | ||||||
| Function / homology |  Function and homology information Function and homology informationdetection of fungus / nitric oxide production involved in inflammatory response / MHC class II biosynthetic process / positive regulation of cellular response to macrophage colony-stimulating factor stimulus / lipopolysaccharide immune receptor activity / positive regulation of nucleotide-binding oligomerization domain containing 1 signaling pathway / positive regulation of matrix metallopeptidase secretion / Toll-like receptor 4 binding / detection of lipopolysaccharide / regulation of dendritic cell cytokine production ...detection of fungus / nitric oxide production involved in inflammatory response / MHC class II biosynthetic process / positive regulation of cellular response to macrophage colony-stimulating factor stimulus / lipopolysaccharide immune receptor activity / positive regulation of nucleotide-binding oligomerization domain containing 1 signaling pathway / positive regulation of matrix metallopeptidase secretion / Toll-like receptor 4 binding / detection of lipopolysaccharide / regulation of dendritic cell cytokine production / lipopolysaccharide receptor complex / MyD88-independent TLR4 cascade / negative regulation of interleukin-23 production / cellular response to oxidised low-density lipoprotein particle stimulus / TRIF-mediated programmed cell death / wound healing involved in inflammatory response / B cell proliferation involved in immune response / nucleotide-binding oligomerization domain containing 1 signaling pathway / positive regulation of nucleotide-binding oligomerization domain containing 2 signaling pathway / positive regulation of stress-activated MAPK cascade / Toll Like Receptor 4 (TLR4) Cascade / intestinal epithelial structure maintenance / Caspase activation via Death Receptors in the presence of ligand / positive regulation of interleukin-1 production / macrophage activation / Regulation of TLR by endogenous ligand / astrocyte development / TRIF-dependent toll-like receptor signaling pathway / microglia differentiation / nucleotide-binding oligomerization domain containing 2 signaling pathway / NAD+ nucleosidase activity, cyclic ADP-ribose generating / positive regulation of MHC class II biosynthetic process / positive regulation of macrophage activation / positive regulation of platelet activation / positive regulation of lipopolysaccharide-mediated signaling pathway / negative regulation of interleukin-17 production / MyD88 deficiency (TLR2/4) / positive regulation of cytokine production involved in inflammatory response / positive regulation of chemokine (C-X-C motif) ligand 2 production / positive regulation of extrinsic apoptotic signaling pathway / IRAK4 deficiency (TLR2/4) / negative regulation of cold-induced thermogenesis / positive regulation of macrophage cytokine production / positive regulation of smooth muscle cell migration / MyD88-dependent toll-like receptor signaling pathway / MyD88:MAL(TIRAP) cascade initiated on plasma membrane / T-helper 1 type immune response / toll-like receptor 4 signaling pathway / toll-like receptor signaling pathway / RSV-host interactions / positive regulation of NLRP3 inflammasome complex assembly / negative regulation of osteoclast differentiation / positive regulation of reactive oxygen species biosynthetic process / cellular response to lipoteichoic acid / negative regulation of interleukin-6 production / negative regulation of type II interferon production / Respiratory syncytial virus (RSV) attachment and entry / positive regulation of interferon-alpha production / positive regulation of interleukin-10 production / negative regulation of tumor necrosis factor production / phagocytosis / phagocytic cup / stress-activated MAPK cascade / positive regulation of chemokine production / JNK cascade / cellular response to platelet-derived growth factor stimulus / ruffle / positive regulation of B cell proliferation / ERK1 and ERK2 cascade / nitric oxide biosynthetic process / positive regulation of interleukin-12 production / Regulation of TBK1, IKKε (IKBKE)-mediated activation of IRF3, IRF7 / IRAK2 mediated activation of TAK1 complex upon TLR7/8 or 9 stimulation / positive regulation of smooth muscle cell proliferation / TRAF6-mediated induction of TAK1 complex within TLR4 complex / positive regulation of interferon-beta production / lipopolysaccharide-mediated signaling pathway / Activation of IRF3, IRF7 mediated by TBK1, IKKε (IKBKE) / IKK complex recruitment mediated by RIP1 / positive regulation of interleukin-1 beta production / positive regulation of interleukin-8 production / positive regulation of JNK cascade / lipopolysaccharide binding / Heme signaling / cellular response to mechanical stimulus / positive regulation of NF-kappaB transcription factor activity / negative regulation of ERK1 and ERK2 cascade / cellular response to type II interferon / positive regulation of interleukin-6 production / positive regulation of type II interferon production / cellular response to amyloid-beta / positive regulation of inflammatory response / positive regulation of nitric oxide biosynthetic process / positive regulation of tumor necrosis factor production / transmembrane signaling receptor activity / signaling receptor activity / amyloid-beta binding / ER-Phagosome pathway / cellular response to lipopolysaccharide / response to lipopolysaccharide Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method | ELECTRON MICROSCOPY / single particle reconstruction / cryo EM / Resolution: 2.7 Å | ||||||
 Authors Authors | Fu, Y. / Kim, H. / Zamyatina, A. / Kim, H.M. | ||||||
| Funding support | 1items
| ||||||
 Citation Citation |  Journal: Nat Commun / Year: 2025 Journal: Nat Commun / Year: 2025Title: Structural insight into TLR4/MD-2 activation by synthetic LPS mimetics with distinct binding modes. Authors: Yaoyao Fu / Hyojin Kim / Dong Sun Lee / Ah-Reum Han / Holger Heine / Alla Zamyatina / Ho Min Kim /    Abstract: The mammalian pattern-recognition receptor TLR4/MD-2 (Toll-like receptor 4/myeloid differentiation factor-2) can be activated by a wide variety of pathogen-associated and endogenous molecules, with ...The mammalian pattern-recognition receptor TLR4/MD-2 (Toll-like receptor 4/myeloid differentiation factor-2) can be activated by a wide variety of pathogen-associated and endogenous molecules, with Gram-negative bacterial lipopolysaccharide (LPS) being the primary natural TLR4 agonist. Activation of TLR4 triggers cellular signaling that enables the beneficial innate immune responses and enhances adaptive immunity, thereby emphasizing the potential of TLR4 agonists for the management of diseases with an immunopathological background and for use as vaccine adjuvants. Given the challenges associated with LPS-derived products, including structural complexity, heterogeneity, toxicity, and species specificity, synthetic molecules targeting TLR4/MD-2 offer a promising alternative. Here, we elucidate the structural basis for the recognition of synthetic LPS-mimicking glycolipids, Disaccharide Lipid A Mimetics (DLAMs), by human and mouse TLR4/MD-2 through cryo-EM structures of six dimeric [TLR4/MD-2/ligand] complexes resolved at 2.2-3.1 Å. We reveal that the specific binding modes of DLAMs, distinct from those of LPS, are essential for the species-independent TLR4 agonistic activity. DLAMs function as a molecular bridge, effectively induce the dimerization of TLR4/MD-2 complexes through specific carbohydrate structure-relevant ligand-protein interactions. Our findings reveal the distinct molecular modes of TLR4 activation, and provide a structural basis for the rationale design and development of innovative, highly potent TLR4-targeting immunotherapeutics and adjuvants. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  9j03.cif.gz 9j03.cif.gz | 280.1 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb9j03.ent.gz pdb9j03.ent.gz | 225.5 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  9j03.json.gz 9j03.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  9j03_validation.pdf.gz 9j03_validation.pdf.gz | 2 MB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  9j03_full_validation.pdf.gz 9j03_full_validation.pdf.gz | 2 MB | Display | |
| Data in XML |  9j03_validation.xml.gz 9j03_validation.xml.gz | 65.8 KB | Display | |
| Data in CIF |  9j03_validation.cif.gz 9j03_validation.cif.gz | 93.9 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/j0/9j03 https://data.pdbj.org/pub/pdb/validation_reports/j0/9j03 ftp://data.pdbj.org/pub/pdb/validation_reports/j0/9j03 ftp://data.pdbj.org/pub/pdb/validation_reports/j0/9j03 | HTTPS FTP |
-Related structure data
| Related structure data |  61047MC  8wo1C  8wqtC  8wryC  8wsaC  8wtaC C: citing same article ( M: map data used to model this data |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
-Protein , 2 types, 4 molecules CDBA
| #1: Protein | Mass: 16385.941 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: LY96 / Cell (production host): High Five / Production host: Homo sapiens (human) / Gene: LY96 / Cell (production host): High Five / Production host:  Trichoplusia ni (cabbage looper) / References: UniProt: B3Y6A6 Trichoplusia ni (cabbage looper) / References: UniProt: B3Y6A6#2: Protein | Mass: 68838.328 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: TLR4 / Cell (production host): High Five / Production host: Homo sapiens (human) / Gene: TLR4 / Cell (production host): High Five / Production host:  Trichoplusia ni (cabbage looper) / References: UniProt: O00206 Trichoplusia ni (cabbage looper) / References: UniProt: O00206 |
|---|
-Sugars , 6 types, 16 molecules 
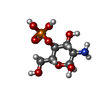

| #3: Polysaccharide | Source method: isolated from a genetically manipulated source #4: Polysaccharide | Source method: isolated from a genetically manipulated source #5: Polysaccharide | #7: Sugar | ChemComp-NAG / #8: Sugar | #9: Sugar | Type: D-saccharide, alpha linking / Mass: 274.162 Da / Num. of mol.: 2 / Source method: obtained synthetically / Formula: C7H15O9P / Feature type: SUBJECT OF INVESTIGATION |
|---|
-Non-polymers , 1 types, 6 molecules 
| #6: Chemical | ChemComp-2IL / ( |
|---|
-Details
| Has ligand of interest | Y |
|---|---|
| Has protein modification | Y |
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method: single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: human TLR4/MD-2/DLAM1 complex / Type: COMPLEX / Entity ID: #1-#2 / Source: RECOMBINANT | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular weight | Experimental value: NO | |||||||||||||||
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) | |||||||||||||||
| Source (recombinant) | Organism:  Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper) | |||||||||||||||
| Buffer solution | pH: 8 | |||||||||||||||
| Buffer component |
| |||||||||||||||
| Specimen | Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: YES | |||||||||||||||
| Specimen support | Details: 10mA / Grid material: COPPER / Grid mesh size: 300 divisions/in. / Grid type: Quantifoil R1.2/1.3 | |||||||||||||||
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: TFS KRIOS |
| Electron gun | Electron source:  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD / Nominal magnification: 105000 X / Calibrated magnification: 58962 X / Nominal defocus max: 1700 nm / Nominal defocus min: 700 nm / Cs: 2.7 mm |
| Specimen holder | Cryogen: NITROGEN |
| Image recording | Electron dose: 67.8 e/Å2 / Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Num. of grids imaged: 1 / Num. of real images: 13308 |
- Processing
Processing
| EM software | Name: PHENIX |
|---|---|
| CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION |
| Symmetry | Point symmetry: C2 (2 fold cyclic) |
| 3D reconstruction | Resolution: 2.7 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 181542 / Symmetry type: POINT |
| Refinement | Highest resolution: 2.7 Å |
 Movie
Movie Controller
Controller








 PDBj
PDBj













