[English] 日本語
 Yorodumi
Yorodumi- PDB-9ey8: Crystal structure of human tyrosinase-related protein 1 (TYRP1) i... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 9ey8 | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal structure of human tyrosinase-related protein 1 (TYRP1) in complex with (s)-amino-L-tyrosine | ||||||
 Components Components | 5,6-dihydroxyindole-2-carboxylic acid oxidase | ||||||
 Keywords Keywords | METAL BINDING PROTEIN / melanogenesis / human tyrosinase / tyrosinase inhibitor | ||||||
| Function / homology |  Function and homology information Function and homology informationacetoacetic acid metabolic process / Melanin biosynthesis / Oxidoreductases; Acting on paired donors, with incorporation or reduction of molecular oxygen; With another compound as one donor, and incorporation of one atom of oxygen into the other donor / tyrosinase activity / positive regulation of melanin biosynthetic process / melanin biosynthetic process / melanocyte differentiation / melanosome organization / melanosome membrane / intracellular vesicle ...acetoacetic acid metabolic process / Melanin biosynthesis / Oxidoreductases; Acting on paired donors, with incorporation or reduction of molecular oxygen; With another compound as one donor, and incorporation of one atom of oxygen into the other donor / tyrosinase activity / positive regulation of melanin biosynthetic process / melanin biosynthetic process / melanocyte differentiation / melanosome organization / melanosome membrane / intracellular vesicle / Regulation of MITF-M-dependent genes involved in pigmentation / clathrin-coated endocytic vesicle membrane / melanosome / oxidoreductase activity / endosome membrane / protein homodimerization activity / metal ion binding / cytoplasm Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.2 Å MOLECULAR REPLACEMENT / Resolution: 2.2 Å | ||||||
 Authors Authors | Ng, Y.M. / Soler-Lopez, M. | ||||||
| Funding support | European Union, 1items
| ||||||
 Citation Citation |  Journal: Chembiochem / Year: 2024 Journal: Chembiochem / Year: 2024Title: Interactions of Phenylalanine Derivatives with Human Tyrosinase: Lessons from Experimental and Theoretical tudies. Authors: Faure, C. / Min Ng, Y. / Belle, C. / Soler-Lopez, M. / Khettabi, L. / Saidi, M. / Berthet, N. / Maresca, M. / Philouze, C. / Rachidi, W. / Reglier, M. / du Moulinet d'Hardemare, A. / Jamet, H. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  9ey8.cif.gz 9ey8.cif.gz | 515.6 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb9ey8.ent.gz pdb9ey8.ent.gz | Display |  PDB format PDB format | |
| PDBx/mmJSON format |  9ey8.json.gz 9ey8.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  9ey8_validation.pdf.gz 9ey8_validation.pdf.gz | 8.3 MB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  9ey8_full_validation.pdf.gz 9ey8_full_validation.pdf.gz | 8.4 MB | Display | |
| Data in XML |  9ey8_validation.xml.gz 9ey8_validation.xml.gz | 80.2 KB | Display | |
| Data in CIF |  9ey8_validation.cif.gz 9ey8_validation.cif.gz | 113.8 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/ey/9ey8 https://data.pdbj.org/pub/pdb/validation_reports/ey/9ey8 ftp://data.pdbj.org/pub/pdb/validation_reports/ey/9ey8 ftp://data.pdbj.org/pub/pdb/validation_reports/ey/9ey8 | HTTPS FTP |
-Related structure data
| Related structure data |  9ey5C  9ey6C  9ey7C C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||||||
| 2 | 
| ||||||||||||
| 3 | 
| ||||||||||||
| 4 | 
| ||||||||||||
| Unit cell |
|
- Components
Components
-Protein , 1 types, 4 molecules AABACADA
| #1: Protein | Mass: 51132.820 Da / Num. of mol.: 4 Source method: isolated from a genetically manipulated source Details: Including three amino acids (ENL) which formed part of the TEV cleavage site Source: (gene. exp.)  Homo sapiens (human) / Gene: TYRP1, CAS2, TYRP, TYRRP / Plasmid: pACEBac1 / Cell (production host): Insect cell / Cell line (production host): Sf21 / Production host: Homo sapiens (human) / Gene: TYRP1, CAS2, TYRP, TYRRP / Plasmid: pACEBac1 / Cell (production host): Insect cell / Cell line (production host): Sf21 / Production host:  References: UniProt: P17643, Oxidoreductases; Acting on paired donors, with incorporation or reduction of molecular oxygen; With another compound as one donor, and incorporation of one atom of oxygen into the other donor |
|---|
-Sugars , 11 types, 28 molecules 
| #2: Polysaccharide | alpha-D-mannopyranose-(1-3)-[alpha-D-mannopyranose-(1-6)]beta-D-mannopyranose-(1-4)-2-acetamido-2- ...alpha-D-mannopyranose-(1-3)-[alpha-D-mannopyranose-(1-6)]beta-D-mannopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-[alpha-L-fucopyranose-(1-6)]2-acetamido-2-deoxy-beta-D-glucopyranose Source method: isolated from a genetically manipulated source #3: Polysaccharide | Source method: isolated from a genetically manipulated source #4: Polysaccharide | Source method: isolated from a genetically manipulated source #5: Polysaccharide | Source method: isolated from a genetically manipulated source #6: Polysaccharide | 2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose Source method: isolated from a genetically manipulated source #7: Polysaccharide | 2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-[alpha-L-fucopyranose-(1-6)]2-acetamido-2-deoxy-beta- ...2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-[alpha-L-fucopyranose-(1-6)]2-acetamido-2-deoxy-beta-D-glucopyranose | Source method: isolated from a genetically manipulated source #8: Polysaccharide | alpha-D-mannopyranose-(1-2)-alpha-D-mannopyranose-(1-3)-[alpha-D-mannopyranose-(1-6)]beta-D- ...alpha-D-mannopyranose-(1-2)-alpha-D-mannopyranose-(1-3)-[alpha-D-mannopyranose-(1-6)]beta-D-mannopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-[alpha-L-fucopyranose-(1-6)]2-acetamido-2-deoxy-beta-D-glucopyranose | Type: oligosaccharide / Mass: 1219.105 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source #9: Polysaccharide | alpha-D-mannopyranose-(1-2)-alpha-D-mannopyranose-(1-3)-[alpha-D-mannopyranose-(1-3)-alpha-D- ...alpha-D-mannopyranose-(1-2)-alpha-D-mannopyranose-(1-3)-[alpha-D-mannopyranose-(1-3)-alpha-D-mannopyranose-(1-6)]beta-D-mannopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose | Source method: isolated from a genetically manipulated source #10: Polysaccharide | alpha-D-mannopyranose-(1-2)-alpha-D-mannopyranose-(1-3)-[alpha-D-mannopyranose-(1-6)]beta-D- ...alpha-D-mannopyranose-(1-2)-alpha-D-mannopyranose-(1-3)-[alpha-D-mannopyranose-(1-6)]beta-D-mannopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose | Source method: isolated from a genetically manipulated source #11: Polysaccharide | Source method: isolated from a genetically manipulated source #12: Sugar | ChemComp-NAG / |
|---|
-Non-polymers , 4 types, 1012 molecules 

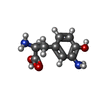




| #13: Chemical | ChemComp-GOL / #14: Chemical | ChemComp-ZN / #15: Chemical | ChemComp-TY2 / | #16: Water | ChemComp-HOH / | |
|---|
-Details
| Has ligand of interest | Y |
|---|---|
| Has protein modification | Y |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.95 Å3/Da / Density % sol: 58.27 % |
|---|---|
| Crystal grow | Temperature: 277 K / Method: vapor diffusion, sitting drop / pH: 7.5 / Details: Sodium bromide, Bis-Tris propane, PEG 3350 |
-Data collection
| Diffraction | Mean temperature: 100 K / Serial crystal experiment: N |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  ESRF ESRF  / Beamline: MASSIF-1 / Wavelength: 0.965459 Å / Beamline: MASSIF-1 / Wavelength: 0.965459 Å |
| Detector | Type: DECTRIS PILATUS3 2M / Detector: PIXEL / Date: Oct 8, 2021 |
| Radiation | Monochromator: C(110) / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.965459 Å / Relative weight: 1 |
| Reflection | Resolution: 2.198→47.26 Å / Num. obs: 234158 / % possible obs: 99.51 % / Redundancy: 3.4 % / Biso Wilson estimate: 40.36 Å2 / CC1/2: 0.996 / CC star: 0.999 / Rmerge(I) obs: 0.0886 / Rpim(I) all: 0.05616 / Rrim(I) all: 0.1052 / Net I/σ(I): 8.29 |
| Reflection shell | Resolution: 2.2→2.22 Å / Redundancy: 2.9 % / Rmerge(I) obs: 1.377 / Mean I/σ(I) obs: 0.59 / Num. unique obs: 6894 / CC1/2: 0.395 / CC star: 0.752 / Rpim(I) all: 0.9268 / % possible all: 88.35 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT / Resolution: 2.2→47.26 Å / SU ML: 0.3077 / Cross valid method: FREE R-VALUE / σ(F): 1.33 / Phase error: 24.079 MOLECULAR REPLACEMENT / Resolution: 2.2→47.26 Å / SU ML: 0.3077 / Cross valid method: FREE R-VALUE / σ(F): 1.33 / Phase error: 24.079 Stereochemistry target values: GeoStd + Monomer Library + CDL v1.2
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.1 Å / Solvent model: FLAT BULK SOLVENT MODEL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 43.57 Å2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.2→47.26 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
|
 Movie
Movie Controller
Controller


 PDBj
PDBj



