[English] 日本語
 Yorodumi
Yorodumi- PDB-9cf5: STRUCTURE OF CD4 MIMETIC CJF-III-288 IN COMPLEX WITH BG505 SOSIP.... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 9cf5 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | STRUCTURE OF CD4 MIMETIC CJF-III-288 IN COMPLEX WITH BG505 SOSIP.664 HIV-1ENV TRIMER AND 17B FAB | |||||||||
 Components Components |
| |||||||||
 Keywords Keywords | VIRAL PROTEIN/IMMUNE SYSTEM / HIV-1 / FUSION / ENTRY / MEMBRANE / VIRAL PROTEIN / VIRAL PROTEIN-IMMUNE SYSTEM COMPLEX | |||||||||
| Function / homology |  Function and homology information Function and homology informationsymbiont-mediated perturbation of host defense response / positive regulation of plasma membrane raft polarization / positive regulation of receptor clustering / host cell endosome membrane / clathrin-dependent endocytosis of virus by host cell / viral protein processing / fusion of virus membrane with host plasma membrane / fusion of virus membrane with host endosome membrane / viral envelope / virion attachment to host cell ...symbiont-mediated perturbation of host defense response / positive regulation of plasma membrane raft polarization / positive regulation of receptor clustering / host cell endosome membrane / clathrin-dependent endocytosis of virus by host cell / viral protein processing / fusion of virus membrane with host plasma membrane / fusion of virus membrane with host endosome membrane / viral envelope / virion attachment to host cell / host cell plasma membrane / virion membrane / structural molecule activity / identical protein binding / membrane Similarity search - Function | |||||||||
| Biological species |   Human immunodeficiency virus 1 Human immunodeficiency virus 1 Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | ELECTRON MICROSCOPY / single particle reconstruction / cryo EM / Resolution: 3.5 Å | |||||||||
 Authors Authors | Niu, L. / Tolbert, W.D. / Pazgier, M. | |||||||||
| Funding support |  United States, 2items United States, 2items
| |||||||||
 Citation Citation |  Journal: bioRxiv / Year: 2024 Journal: bioRxiv / Year: 2024Title: The asymmetric opening of HIV-1 Env by a potent CD4 mimetic enables anti-coreceptor binding site antibodies to mediate ADCC. Authors: Jonathan Richard / Michael W Grunst / Ling Niu / Marco A Díaz-Salinas / William D Tolbert / Lorie Marchitto / Fei Zhou / Catherine Bourassa / Derek Yang / Ta Jung Chiu / Hung-Ching Chen / ...Authors: Jonathan Richard / Michael W Grunst / Ling Niu / Marco A Díaz-Salinas / William D Tolbert / Lorie Marchitto / Fei Zhou / Catherine Bourassa / Derek Yang / Ta Jung Chiu / Hung-Ching Chen / Mehdi Benlarbi / / Suneetha Gottumukkala / Wenwei Li / Katrina Dionne / Étienne Bélanger / Debashree Chatterjee / Halima Medjahed / Wayne A Hendrickson / Joseph Sodroski / Zabrina C Lang / Abraham J Morton / Rick K Huang / Doreen Matthies / Amos B Smith / Walther Mothes / James B Munro / Marzena Pazgier / Andrés Finzi /   Abstract: HIV-1 envelope glycoproteins (Env) from primary HIV-1 isolates typically adopt a pretriggered "closed" conformation that resists to CD4-induced (CD4i) non-neutralizing antibodies (nnAbs) mediating ...HIV-1 envelope glycoproteins (Env) from primary HIV-1 isolates typically adopt a pretriggered "closed" conformation that resists to CD4-induced (CD4i) non-neutralizing antibodies (nnAbs) mediating antibody-dependent cellular cytotoxicity (ADCC). CD4-mimetic compounds (CD4mcs) "open-up" Env allowing binding of CD4i nnAbs, thereby sensitizing HIV-1-infected cells to ADCC. Two families of CD4i nnAbs, the anti-cluster A and anti-coreceptor binding site (CoRBS) Abs, are required to mediate ADCC in combination with the indane CD4mc BNM-III-170. Recently, new indoline CD4mcs with improved potency and breadth have been described. Here, we show that the lead indoline CD4mc, CJF-III-288, sensitizes HIV-1-infected cells to ADCC mediated by anti-CoRBS Abs alone, contributing to improved ADCC activity. Structural and conformational analyses reveal that CJF-III-288, in combination with anti-CoRBS Abs, potently stabilizes an asymmetric "open" State-3 Env conformation, This Env conformation orients the anti-CoRBS Ab to improve ADCC activity and therapeutic potential. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  9cf5.cif.gz 9cf5.cif.gz | 431.7 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb9cf5.ent.gz pdb9cf5.ent.gz | 340.8 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  9cf5.json.gz 9cf5.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/cf/9cf5 https://data.pdbj.org/pub/pdb/validation_reports/cf/9cf5 ftp://data.pdbj.org/pub/pdb/validation_reports/cf/9cf5 ftp://data.pdbj.org/pub/pdb/validation_reports/cf/9cf5 | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  45530MC M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
-Envelope glycoprotein ... , 2 types, 6 molecules ACEBDF
| #1: Protein | Mass: 53864.086 Da / Num. of mol.: 3 Mutation: T332N, A501C, E509R, K510R, insertion of RR after residue 511 Source method: isolated from a genetically manipulated source Details: BG505 SOSIP.664 construct / Source: (gene. exp.)   Human immunodeficiency virus 1 / Gene: env / Cell line (production host): HEK 293 GNT1- / Production host: Human immunodeficiency virus 1 / Gene: env / Cell line (production host): HEK 293 GNT1- / Production host:  Homo sapiens (human) / References: UniProt: Q2N0S6 Homo sapiens (human) / References: UniProt: Q2N0S6#2: Protein | Mass: 17146.482 Da / Num. of mol.: 3 / Fragment: residues 512-664 (509-661 Uniprot numbering) / Mutation: I559P, T605C Source method: isolated from a genetically manipulated source Details: BG505 SOSIP.664 construct / Source: (gene. exp.)   Human immunodeficiency virus 1 / Gene: env / Cell line (production host): HEK 293 GNT1- / Production host: Human immunodeficiency virus 1 / Gene: env / Cell line (production host): HEK 293 GNT1- / Production host:  Homo sapiens (human) / References: UniProt: Q2N0S6 Homo sapiens (human) / References: UniProt: Q2N0S6 |
|---|
-Antibody , 2 types, 6 molecules KIGLJH
| #3: Antibody | Mass: 23399.898 Da / Num. of mol.: 3 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Cell line (production host): HEK293 / Production host: Homo sapiens (human) / Cell line (production host): HEK293 / Production host:  Homo sapiens (human) Homo sapiens (human)#4: Antibody | Mass: 24572.479 Da / Num. of mol.: 3 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Cell line (production host): HEK293 / Production host: Homo sapiens (human) / Cell line (production host): HEK293 / Production host:  Homo sapiens (human) Homo sapiens (human) |
|---|
-Sugars , 4 types, 38 molecules 
| #5: Polysaccharide | 2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose Source method: isolated from a genetically manipulated source #6: Polysaccharide | alpha-D-mannopyranose-(1-2)-alpha-D-mannopyranose-(1-3)-[alpha-D-mannopyranose-(1-6)]beta-D- ...alpha-D-mannopyranose-(1-2)-alpha-D-mannopyranose-(1-3)-[alpha-D-mannopyranose-(1-6)]beta-D-mannopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose | Source method: isolated from a genetically manipulated source #7: Polysaccharide | Source method: isolated from a genetically manipulated source #8: Sugar | ChemComp-NAG / |
|---|
-Non-polymers , 1 types, 3 molecules 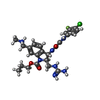
| #9: Chemical |
|---|
-Details
| Has ligand of interest | Y |
|---|---|
| Has protein modification | Y |
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method: single particle reconstruction |
- Sample preparation
Sample preparation
| Component |
| ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular weight | Experimental value: NO | ||||||||||||||||||||||||
| Source (natural) |
| ||||||||||||||||||||||||
| Source (recombinant) |
| ||||||||||||||||||||||||
| Buffer solution | pH: 7.2 | ||||||||||||||||||||||||
| Specimen | Conc.: 1.5 mg/ml / Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: YES | ||||||||||||||||||||||||
| Specimen support | Grid material: COPPER / Grid mesh size: 400 divisions/in. / Grid type: Quantifoil R1.2/1.3 | ||||||||||||||||||||||||
| Vitrification | Instrument: FEI VITROBOT MARK IV / Cryogen name: ETHANE |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TITAN KRIOS |
| Electron gun | Electron source:  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: SPOT SCAN FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: SPOT SCAN |
| Electron lens | Mode: BRIGHT FIELD / Nominal defocus max: 2700 nm / Nominal defocus min: 500 nm / Cs: 2.7 mm |
| Specimen holder | Cryogen: NITROGEN / Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER |
| Image recording | Electron dose: 54.2 e/Å2 / Film or detector model: GATAN K3 (6k x 4k) / Num. of grids imaged: 1 / Num. of real images: 9431 |
- Processing
Processing
| EM software |
| ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION | ||||||||||||||||||||||||
| Symmetry | Point symmetry: C1 (asymmetric) | ||||||||||||||||||||||||
| 3D reconstruction | Resolution: 3.5 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 1087694 / Symmetry type: POINT | ||||||||||||||||||||||||
| Atomic model building | PDB-ID: 7LOK Accession code: 7LOK / Source name: PDB / Type: experimental model | ||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller



 PDBj
PDBj








