[English] 日本語
 Yorodumi
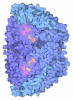
Yorodumi- PDB-8s7v: Methyl-coenzyme M reductase activation complex binding to the A2 ... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 8s7v | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Methyl-coenzyme M reductase activation complex binding to the A2 component | |||||||||||||||||||||||||||||||||||||||||||||||||||
 Components Components |
| |||||||||||||||||||||||||||||||||||||||||||||||||||
 Keywords Keywords | OXIDOREDUCTASE / Methyl-coenzyme M reductase / activation complex / ATPase / Iron-sulfur clusters | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Function / homology |  Function and homology information Function and homology informationorganic phosphonate catabolic process / coenzyme-B sulfoethylthiotransferase / coenzyme-B sulfoethylthiotransferase activity / methanogenesis / ATP hydrolysis activity / ATP binding / metal ion binding / cytoplasm Similarity search - Function | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological species |  Methanococcus maripaludis (archaea) Methanococcus maripaludis (archaea) | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Method | ELECTRON MICROSCOPY / single particle reconstruction / cryo EM / Resolution: 2.56 Å | |||||||||||||||||||||||||||||||||||||||||||||||||||
 Authors Authors | Ramirez-Amador, F. / Paul, S. / Kumar, A. / Schuller, J.M. | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Funding support | European Union, 1items
| |||||||||||||||||||||||||||||||||||||||||||||||||||
 Citation Citation |  Journal: Nature / Year: 2025 Journal: Nature / Year: 2025Title: Structure of the ATP-driven methyl-coenzyme M reductase activation complex. Authors: Fidel Ramírez-Amador / Sophia Paul / Anuj Kumar / Christian Lorent / Sebastian Keller / Stefan Bohn / Thinh Nguyen / Stefano Lometto / Dennis Vlegels / Jörg Kahnt / Darja Deobald / Frank ...Authors: Fidel Ramírez-Amador / Sophia Paul / Anuj Kumar / Christian Lorent / Sebastian Keller / Stefan Bohn / Thinh Nguyen / Stefano Lometto / Dennis Vlegels / Jörg Kahnt / Darja Deobald / Frank Abendroth / Olalla Vázquez / Georg Hochberg / Silvan Scheller / Sven T Stripp / Jan Michael Schuller /   Abstract: Methyl-coenzyme M reductase (MCR) is the enzyme responsible for nearly all biologically generated methane. Its active site comprises coenzyme F, a porphyrin-based cofactor with a central nickel ion ...Methyl-coenzyme M reductase (MCR) is the enzyme responsible for nearly all biologically generated methane. Its active site comprises coenzyme F, a porphyrin-based cofactor with a central nickel ion that is active exclusively in the Ni(I) state. How methanogenic archaea perform the reductive activation of F represents a major gap in our understanding of one of the most ancient bioenergetic systems in nature. Here we purified and characterized the MCR activation complex from Methanococcus maripaludis. McrC, a small subunit encoded in the mcr operon, co-purifies with the methanogenic marker proteins Mmp7, Mmp17, Mmp3 and the A2 component. We demonstrated that this complex can activate MCR in vitro in a strictly ATP-dependent manner, enabling the formation of methane. In addition, we determined the cryo-electron microscopy structure of the MCR activation complex exhibiting different functional states with local resolutions reaching 1.8-2.1 Å. Our data revealed three complex iron-sulfur clusters that formed an electron transfer pathway towards F. Topology and electron paramagnetic resonance spectroscopy analyses indicate that these clusters are similar to the [8Fe-9S-C] cluster, a maturation intermediate of the catalytic cofactor in nitrogenase. Altogether, our findings offer insights into the activation mechanism of MCR and prospects on the early evolution of nitrogenase. | |||||||||||||||||||||||||||||||||||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  8s7v.cif.gz 8s7v.cif.gz | 811.3 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb8s7v.ent.gz pdb8s7v.ent.gz | 654.7 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  8s7v.json.gz 8s7v.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/s7/8s7v https://data.pdbj.org/pub/pdb/validation_reports/s7/8s7v ftp://data.pdbj.org/pub/pdb/validation_reports/s7/8s7v ftp://data.pdbj.org/pub/pdb/validation_reports/s7/8s7v | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  19787MC  8s7xC  9h1lC M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
-Methyl-coenzyme M reductase subunit ... , 3 types, 6 molecules ABEDCF
| #1: Protein | Mass: 29665.488 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Details: Methyl-coenzyme M reductase subunit gamma / Source: (natural)  Methanococcus maripaludis (archaea) Methanococcus maripaludis (archaea)References: UniProt: A0A2L1CBG2, coenzyme-B sulfoethylthiotransferase #2: Protein | Mass: 46701.203 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Details: Methyl-coenzyme M reductase subunit beta / Source: (natural)  Methanococcus maripaludis (archaea) Methanococcus maripaludis (archaea)References: UniProt: A0A2L1CBB3, coenzyme-B sulfoethylthiotransferase #3: Protein | Mass: 61230.703 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Details: Methyl-coenzyme M reductase subunit alpha / Source: (natural)  Methanococcus maripaludis (archaea) Methanococcus maripaludis (archaea)References: UniProt: A0A2L1CBB0, coenzyme-B sulfoethylthiotransferase |
|---|
-Methanogenesis marker protein ... , 2 types, 2 molecules GH
| #4: Protein | Mass: 21119.539 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Details: Methanogenesis marker protein 17 / Source: (natural)  Methanococcus maripaludis (archaea) / References: UniProt: G0H411 Methanococcus maripaludis (archaea) / References: UniProt: G0H411 |
|---|---|
| #5: Protein | Mass: 35024.746 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Details: Methanogenesis marker protein 7 / Source: (natural)  Methanococcus maripaludis (archaea) / References: UniProt: G0H350 Methanococcus maripaludis (archaea) / References: UniProt: G0H350 |
-Protein , 4 types, 4 molecules IKJL
| #6: Protein | Mass: 24906.715 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Details: Methyl-coenzyme M reductase operon protein C / Source: (gene. exp.)  Methanococcus maripaludis (archaea) / Gene: GYY_08635 / Production host: Methanococcus maripaludis (archaea) / Gene: GYY_08635 / Production host:  Methanococcus maripaludis (archaea) / Strain (production host): JJ -upt / References: UniProt: G0H3B1 Methanococcus maripaludis (archaea) / Strain (production host): JJ -upt / References: UniProt: G0H3B1 |
|---|---|
| #7: Protein | Mass: 59572.562 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Details: Methyl-coenzyme M reductase system, component A2 / Source: (natural)  Methanococcus maripaludis (archaea) / References: UniProt: A0A2L1C9A1 Methanococcus maripaludis (archaea) / References: UniProt: A0A2L1C9A1 |
| #8: Protein | Mass: 56520.801 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Details: Methanogenesis marker protein 3 / Source: (natural)  Methanococcus maripaludis (archaea) / References: UniProt: A9A8E0 Methanococcus maripaludis (archaea) / References: UniProt: A9A8E0 |
| #9: Protein | Mass: 10640.849 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Details: DUF2098 domain-containing protein / Source: (natural)  Methanococcus maripaludis (archaea) / References: UniProt: A0A2L1CAX0 Methanococcus maripaludis (archaea) / References: UniProt: A0A2L1CAX0 |
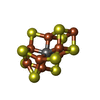
-Non-polymers , 8 types, 13 molecules 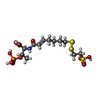













| #10: Chemical | ChemComp-SHT / | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| #11: Chemical | ChemComp-TP7 / | ||||||||
| #12: Chemical | ChemComp-COM / | ||||||||
| #13: Chemical | | #14: Chemical | #15: Chemical | ChemComp-ZN / | #16: Chemical | #17: Chemical | |
-Details
| Has ligand of interest | Y |
|---|---|
| Has protein modification | Y |
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: 3D ARRAY / 3D reconstruction method: single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: Methyl-coenzyme M reductase activation complex binding to A2 component Type: COMPLEX / Entity ID: #1, #3, #2, #4-#6, #8, #7, #9 / Source: NATURAL | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular weight | Value: 0.48 MDa / Experimental value: YES | |||||||||||||||
| Source (natural) | Organism:  Methanococcus maripaludis (archaea) Methanococcus maripaludis (archaea) | |||||||||||||||
| Buffer solution | pH: 7.6 | |||||||||||||||
| Buffer component |
| |||||||||||||||
| Specimen | Conc.: 1.25 mg/ml / Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: YES | |||||||||||||||
| Specimen support | Details: 15 mA / Grid material: COPPER / Grid mesh size: 300 divisions/in. / Grid type: Quantifoil R1.2/1.3 | |||||||||||||||
| Vitrification | Instrument: FEI VITROBOT MARK IV / Cryogen name: ETHANE-PROPANE / Humidity: 100 % / Chamber temperature: 277 K Details: Vitrification setup inside a Coy Lab's Vinyl Anaerobic Chamber to preserve the protein sample under strict anaerobic conditions (95% O2 / 5% H2). |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: TFS KRIOS |
| Electron gun | Electron source:  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD / Nominal magnification: 165000 X / Nominal defocus max: 2000 nm / Nominal defocus min: 500 nm / Cs: 2.7 mm |
| Image recording | Electron dose: 60 e/Å2 / Film or detector model: FEI FALCON IV (4k x 4k) / Num. of real images: 17548 Details: 7781 micrographs with a 20 deg pretilt to overcome preferred orientation problems |
- Processing
Processing
| EM software |
| ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION | ||||||||||||||||||||||||||||||||||||
| Particle selection | Num. of particles selected: 1557902 Details: 1023667 from non tilted and 534235 from pretilted datasets | ||||||||||||||||||||||||||||||||||||
| 3D reconstruction | Resolution: 2.56 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 484016 / Symmetry type: POINT | ||||||||||||||||||||||||||||||||||||
| Atomic model building | Protocol: AB INITIO MODEL / Space: REAL Details: Rigid body fitting in Chimera and Coot. Further real-space refinement in Phenix. | ||||||||||||||||||||||||||||||||||||
| Atomic model building | Source name: AlphaFold / Type: in silico model |
 Movie
Movie Controller
Controller




 PDBj
PDBj