[English] 日本語
 Yorodumi
Yorodumi- PDB-8rr0: CryoEM structure of Molybdenum bispyranopterin guanine dinucleoti... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 8rr0 | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | CryoEM structure of Molybdenum bispyranopterin guanine dinucleotide formate dehydrogenases ForCE1 from Bacillus subtilis | ||||||||||||||||||||||||
 Components Components |
| ||||||||||||||||||||||||
 Keywords Keywords | OXIDOREDUCTASE / Bacterial metabolism Bioenergetics Metalloenzyme Quinone Iron-Sulfur Cluster Helical Membrane Plug-in | ||||||||||||||||||||||||
| Function / homology |  Function and homology information Function and homology informationformate metabolic process / formate dehydrogenase (NAD+) activity / Oxidoreductases / molybdopterin cofactor binding / respiratory electron transport chain / 2 iron, 2 sulfur cluster binding / 4 iron, 4 sulfur cluster binding / metal ion binding / membrane Similarity search - Function | ||||||||||||||||||||||||
| Biological species |  | ||||||||||||||||||||||||
| Method | ELECTRON MICROSCOPY / single particle reconstruction / cryo EM / Resolution: 3.35 Å | ||||||||||||||||||||||||
 Authors Authors | Cherrier, M.V. / Arnoux, P. / Martin, L. / Nicolet, Y. / Schoehn, G. / Legrand, P. / Broc, M. / Seduk, F. / Brasseur, G. / Arias-Cartin, R. ...Cherrier, M.V. / Arnoux, P. / Martin, L. / Nicolet, Y. / Schoehn, G. / Legrand, P. / Broc, M. / Seduk, F. / Brasseur, G. / Arias-Cartin, R. / Magalon, A. / Walburger, A. / Uzel, A. / Guigliarelli, B. / Grimaldi, S. / Pierrel, F. / Mate, M. | ||||||||||||||||||||||||
| Funding support |  France, 7items France, 7items
| ||||||||||||||||||||||||
 Citation Citation |  Journal: Nat Struct Mol Biol / Year: 2025 Journal: Nat Struct Mol Biol / Year: 2025Title: A scaffold for quinone channeling between membrane and soluble bacterial oxidoreductases. Authors: M Broc / M V Cherrier / A Uzel / R Arias-Cartin / P Arnoux / G Brasseur / F Seduk / B Guigliarelli / P Legrand / F Pierrel / G Schoehn / M J Maté / L Martin / S Grimaldi / Y Nicolet / A Magalon / A Walburger /  Abstract: Redox processes are at the heart of energetic metabolism that drives life on earth. By extension, complex and efficient electron transfer wires are necessary to connect the various metabolic pathways ...Redox processes are at the heart of energetic metabolism that drives life on earth. By extension, complex and efficient electron transfer wires are necessary to connect the various metabolic pathways that are often located in distinct cellular compartments. Here, we uncovered a structural module that enables channeling of quinones from the membrane to various water-soluble redox catalytic units in prokaryotes. Using X-ray crystallography and cryo-electron microscopy, we determined the structure of the unusual bacterial formate dehydrogenase ForCE that contains four ForC catalytic subunits docked around a membrane-associated tetrameric ForE central scaffold. In the latter, a conserved domain that we propose to name helical membrane plugin (HMP) was identified as essential to link formate oxidation, in Bacillus subtilis, to the aerobic respiratory chain. Our bioinformatic analysis indicates that this HMP is associated with different quinone-reducing oxidoreductases, highlighting its broad importance as a functional unit to wire electrons between a given catalytic redox center and the quinone pool. | ||||||||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  8rr0.cif.gz 8rr0.cif.gz | 922.6 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb8rr0.ent.gz pdb8rr0.ent.gz | Display |  PDB format PDB format | |
| PDBx/mmJSON format |  8rr0.json.gz 8rr0.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/rr/8rr0 https://data.pdbj.org/pub/pdb/validation_reports/rr/8rr0 ftp://data.pdbj.org/pub/pdb/validation_reports/rr/8rr0 ftp://data.pdbj.org/pub/pdb/validation_reports/rr/8rr0 | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  19452MC  8rqzC  9gzqC M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
-Protein , 2 types, 8 molecules ACEGBDFH
| #1: Protein | Mass: 21308.650 Da / Num. of mol.: 4 / Source method: isolated from a natural source Source: (natural)  References: UniProt: O34681 #2: Protein | Mass: 109924.477 Da / Num. of mol.: 4 / Source method: isolated from a natural source Source: (natural)  References: UniProt: O34720, Oxidoreductases |
|---|
-Non-polymers , 12 types, 176 molecules 
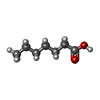



















| #3: Chemical | ChemComp-11A / #4: Chemical | ChemComp-A1H2V / [( Mass: 751.023 Da / Num. of mol.: 12 / Source method: obtained synthetically / Formula: C40H79O10P #5: Chemical | ChemComp-SHV / #6: Chemical | ChemComp-KNA / #7: Chemical | ChemComp-MYR / #8: Chemical | ChemComp-FES / #9: Chemical | ChemComp-SF4 / #10: Chemical | ChemComp-MGD / #11: Chemical | ChemComp-4MO / #12: Chemical | ChemComp-H2S / #13: Chemical | ChemComp-MQ7 / #14: Water | ChemComp-HOH / | |
|---|
-Details
| Has ligand of interest | Y |
|---|---|
| Has protein modification | N |
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method: single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: Molybdenum bispyranopterin guanine dinucleotide formate dehydrogenases ForCE1 Type: COMPLEX / Entity ID: #1-#2 / Source: NATURAL | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular weight | Value: 0.524244 MDa / Experimental value: NO | |||||||||||||||
| Source (natural) | Organism:  | |||||||||||||||
| Buffer solution | pH: 6 / Details: 50 mM MES pH 6 50mM Na2SO4 | |||||||||||||||
| Buffer component |
| |||||||||||||||
| Specimen | Conc.: 0.3 mg/ml / Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: YES | |||||||||||||||
| Specimen support | Details: 30 mA / Grid material: COPPER/RHODIUM / Grid mesh size: 300 divisions/in. / Grid type: Quantifoil R2/1 | |||||||||||||||
| Vitrification | Instrument: FEI VITROBOT MARK IV / Cryogen name: PROPANE / Humidity: 100 % / Chamber temperature: 298 K |
- Electron microscopy imaging
Electron microscopy imaging
| Microscopy | Model: TFS GLACIOS |
|---|---|
| Electron gun | Electron source:  FIELD EMISSION GUN / Accelerating voltage: 200 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 200 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD / Nominal magnification: 45000 X / Nominal defocus max: 3500 nm / Nominal defocus min: 500 nm |
| Image recording | Electron dose: 40 e/Å2 / Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Num. of grids imaged: 1 / Num. of real images: 4977 |
| Image scans | Movie frames/image: 40 / Used frames/image: 2-40 |
- Processing
Processing
| EM software |
| ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION | ||||||||||||||||||||||||||||||||
| Symmetry | Point symmetry: C4 (4 fold cyclic) | ||||||||||||||||||||||||||||||||
| 3D reconstruction | Resolution: 3.35 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 112095 / Algorithm: BACK PROJECTION / Symmetry type: POINT | ||||||||||||||||||||||||||||||||
| Atomic model building | Protocol: FLEXIBLE FIT / Space: RECIPROCAL | ||||||||||||||||||||||||||||||||
| Atomic model building | PDB-ID: 8RQZ Accession code: 8RQZ / Source name: PDB / Type: experimental model |
 Movie
Movie Controller
Controller


 PDBj
PDBj



























