+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 8qcq | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | B. subtilis ApdA-stalled ribosomal complex | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Components Components |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Keywords Keywords | RIBOSOME / Stalling / Nascent chain / elongation arrest / regulation | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Function / homology |  Function and homology information Function and homology informationpositive regulation of rRNA processing / nucleoid / rRNA processing / large ribosomal subunit / transferase activity / ribosomal small subunit assembly / ribosomal large subunit assembly / 5S rRNA binding / small ribosomal subunit / small ribosomal subunit rRNA binding ...positive regulation of rRNA processing / nucleoid / rRNA processing / large ribosomal subunit / transferase activity / ribosomal small subunit assembly / ribosomal large subunit assembly / 5S rRNA binding / small ribosomal subunit / small ribosomal subunit rRNA binding / cytosolic small ribosomal subunit / large ribosomal subunit rRNA binding / cytosolic large ribosomal subunit / cytoplasmic translation / tRNA binding / negative regulation of translation / rRNA binding / structural constituent of ribosome / ribosome / translation / ribonucleoprotein complex / response to antibiotic / mRNA binding / DNA binding / RNA binding / zinc ion binding / metal ion binding / cytoplasm / cytosol Similarity search - Function | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological species |  Amycolatopsis japonica (bacteria) Amycolatopsis japonica (bacteria) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Method | ELECTRON MICROSCOPY / single particle reconstruction / cryo EM / Resolution: 2.3 Å | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Authors Authors | Morici, M. / Wilson, D.N. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Funding support |  Germany, 1items Germany, 1items
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2024 Journal: Nat Commun / Year: 2024Title: RAPP-containing arrest peptides induce translational stalling by short circuiting the ribosomal peptidyltransferase activity. Authors: Martino Morici / Sara Gabrielli / Keigo Fujiwara / Helge Paternoga / Bertrand Beckert / Lars V Bock / Shinobu Chiba / Daniel N Wilson /   Abstract: Arrest peptides containing RAPP (ArgAlaProPro) motifs have been discovered in both Gram-positive and Gram-negative bacteria, where they are thought to regulate expression of important protein ...Arrest peptides containing RAPP (ArgAlaProPro) motifs have been discovered in both Gram-positive and Gram-negative bacteria, where they are thought to regulate expression of important protein localization machinery components. Here we determine cryo-EM structures of ribosomes stalled on RAPP arrest motifs in both Bacillus subtilis and Escherichia coli. Together with molecular dynamics simulations, our structures reveal that the RAPP motifs allow full accommodation of the A-site tRNA, but prevent the subsequent peptide bond from forming. Our data support a model where the RAP in the P-site interacts and stabilizes a single hydrogen atom on the Pro-tRNA in the A-site, thereby preventing an optimal geometry for the nucleophilic attack required for peptide bond formation to occur. This mechanism to short circuit the ribosomal peptidyltransferase activity is likely to operate for the majority of other RAPP-like arrest peptides found across diverse bacterial phylogenies. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  8qcq.cif.gz 8qcq.cif.gz | 3.4 MB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb8qcq.ent.gz pdb8qcq.ent.gz | Display |  PDB format PDB format | |
| PDBx/mmJSON format |  8qcq.json.gz 8qcq.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/qc/8qcq https://data.pdbj.org/pub/pdb/validation_reports/qc/8qcq ftp://data.pdbj.org/pub/pdb/validation_reports/qc/8qcq ftp://data.pdbj.org/pub/pdb/validation_reports/qc/8qcq | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  18332MC  8qbtC M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
+50S ribosomal protein ... , 21 types, 21 molecules 01234CDEFJKLMNOPRSUXY
-Large ribosomal subunit protein ... , 6 types, 6 molecules 6GQTWZ
| #6: Protein | Mass: 7330.452 Da / Num. of mol.: 1 / Source method: isolated from a natural source Source: (natural)  References: UniProt: Q03223 |
|---|---|
| #13: Protein | Mass: 19543.389 Da / Num. of mol.: 1 / Source method: isolated from a natural source Source: (natural)  References: UniProt: P46898 |
| #21: Protein | Mass: 13669.189 Da / Num. of mol.: 1 / Source method: isolated from a natural source Source: (natural)  References: UniProt: P55873 |
| #24: Protein | Mass: 10978.813 Da / Num. of mol.: 1 / Source method: isolated from a natural source Source: (natural)  References: UniProt: P42924 |
| #26: Protein | Mass: 10391.855 Da / Num. of mol.: 1 / Source method: isolated from a natural source Source: (natural)  References: UniProt: P05657 |
| #29: Protein | Mass: 6650.795 Da / Num. of mol.: 1 / Source method: isolated from a natural source Source: (natural)  References: UniProt: P19947 |
-RNA chain , 5 types, 6 molecules ABayw8
| #7: RNA chain | Mass: 948733.750 Da / Num. of mol.: 1 / Source method: isolated from a natural source Source: (natural)  References: GenBank: 728882887 | ||
|---|---|---|---|
| #8: RNA chain | Mass: 38423.863 Da / Num. of mol.: 1 / Source method: isolated from a natural source Source: (natural)  References: GenBank: 1430100595 | ||
| #30: RNA chain | Mass: 503369.125 Da / Num. of mol.: 1 / Source method: isolated from a natural source Source: (natural)  References: GenBank: 225184640 | ||
| #37: RNA chain | Mass: 24832.715 Da / Num. of mol.: 2 / Source method: isolated from a natural source Source: (natural)  References: GenBank: 1837880844 #38: RNA chain | | Mass: 1788.101 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Amycolatopsis japonica (bacteria) Amycolatopsis japonica (bacteria)Production host:  |
-30S ribosomal protein ... , 14 types, 14 molecules ekloqtijmnshrf
| #31: Protein | Mass: 17650.625 Da / Num. of mol.: 1 / Source method: isolated from a natural source Source: (natural)  References: UniProt: P21467 |
|---|---|
| #32: Protein | Mass: 13952.000 Da / Num. of mol.: 1 / Source method: isolated from a natural source Source: (natural)  References: UniProt: P04969 |
| #33: Protein | Mass: 15248.736 Da / Num. of mol.: 1 / Source method: isolated from a natural source Source: (natural)  References: UniProt: P21472 |
| #34: Protein | Mass: 10597.224 Da / Num. of mol.: 1 / Source method: isolated from a natural source Source: (natural)  References: UniProt: P21473 |
| #35: Protein | Mass: 10220.979 Da / Num. of mol.: 1 / Source method: isolated from a natural source Source: (natural)  References: UniProt: P12874 |
| #36: Protein | Mass: 9622.217 Da / Num. of mol.: 1 / Source method: isolated from a natural source Source: (natural)  References: UniProt: P21477 |
| #41: Protein | Mass: 14335.504 Da / Num. of mol.: 1 / Source method: isolated from a natural source Source: (natural)  References: UniProt: P21470 |
| #42: Protein | Mass: 11687.661 Da / Num. of mol.: 1 / Source method: isolated from a natural source Source: (natural)  References: UniProt: P21471 |
| #43: Protein | Mass: 13818.085 Da / Num. of mol.: 1 / Source method: isolated from a natural source Source: (natural)  References: UniProt: P20282 |
| #44: Protein | Mass: 7263.803 Da / Num. of mol.: 1 / Source method: isolated from a natural source Source: (natural)  References: UniProt: P12878 |
| #45: Protein | Mass: 10607.309 Da / Num. of mol.: 1 / Source method: isolated from a natural source Source: (natural)  References: UniProt: P21476 |
| #46: Protein | Mass: 14901.427 Da / Num. of mol.: 1 / Source method: isolated from a natural source Source: (natural)  References: UniProt: P12879 |
| #47: Protein | Mass: 8990.613 Da / Num. of mol.: 1 / Source method: isolated from a natural source Source: (natural)  References: UniProt: P21475 |
| #48: Protein | Mass: 11140.548 Da / Num. of mol.: 1 / Source method: isolated from a natural source Source: (natural)  References: UniProt: P21468 |
-Small ribosomal subunit protein ... , 2 types, 2 molecules cg
| #39: Protein | Mass: 23050.357 Da / Num. of mol.: 1 / Source method: isolated from a natural source Source: (natural)  References: UniProt: P21465 |
|---|---|
| #40: Protein | Mass: 17915.879 Da / Num. of mol.: 1 / Source method: isolated from a natural source Source: (natural)  References: UniProt: P21469 |
-Protein/peptide , 1 types, 1 molecules 7
| #49: Protein/peptide | Mass: 970.128 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Amycolatopsis japonica (bacteria) Amycolatopsis japonica (bacteria)Production host:  |
|---|
-Non-polymers , 5 types, 1270 molecules 


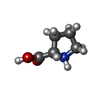





| #50: Chemical | ChemComp-ZN / #51: Chemical | ChemComp-MG / #52: Chemical | ChemComp-K / #53: Chemical | ChemComp-PRO / | #54: Water | ChemComp-HOH / | |
|---|
-Details
| Has ligand of interest | N |
|---|---|
| Has protein modification | Y |
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method: single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: B. subtilis ApdA-stalled ribosomal complex / Type: RIBOSOME / Entity ID: #3-#4, #8-#43, #45-#49 / Source: RECOMBINANT |
|---|---|
| Source (natural) | Organism:  Amycolatopsis japonica (bacteria) Amycolatopsis japonica (bacteria) |
| Source (recombinant) | Organism:  |
| Buffer solution | pH: 7.4 |
| Specimen | Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: YES |
| Vitrification | Cryogen name: ETHANE-PROPANE |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: TFS KRIOS |
| Electron gun | Electron source:  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD / Nominal defocus max: 1800 nm / Nominal defocus min: 600 nm |
| Image recording | Electron dose: 75.6 e/Å2 / Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) |
- Processing
Processing
| EM software | Name: REFMAC / Version: 5.8.0405 / Category: model refinement | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3D reconstruction | Resolution: 2.3 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 142978 / Symmetry type: POINT | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement | Resolution: 2.3→2.3 Å / Cor.coef. Fo:Fc: 0.931 / SU B: 5.999 / SU ML: 0.129 / ESU R: 0.138 Stereochemistry target values: MAXIMUM LIKELIHOOD WITH PHASES Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Solvent model: PARAMETERS FOR MASK CACLULATION | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 83.844 Å2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: 1 / Total: 136962 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller






 PDBj
PDBj

































