[English] 日本語
 Yorodumi
Yorodumi- PDB-8pdh: The phosphatase and C2 domains of SHIP1 with covalent Z1742148362 -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 8pdh | ||||||
|---|---|---|---|---|---|---|---|
| Title | The phosphatase and C2 domains of SHIP1 with covalent Z1742148362 | ||||||
 Components Components | Phosphatidylinositol 3,4,5-trisphosphate 5-phosphatase 1 | ||||||
 Keywords Keywords | HYDROLASE / ligand / phoshphatase / C2 | ||||||
| Function / homology |  Function and homology information Function and homology informationinositol-polyphosphate 5-phosphatase / negative regulation of neutrophil differentiation / inositol-1,3,4,5-tetrakisphosphate 5-phosphatase activity / inositol-polyphosphate 5-phosphatase activity / phosphatidylinositol-3,4,5-trisphosphate 3-phosphatase activity / phosphatidylinositol-3,4,5-trisphosphate 5-phosphatase / phosphoinositide 5-phosphatase / phosphatidylinositol-3,4,5-trisphosphate 5-phosphatase activity / phosphatidylinositol-4,5-bisphosphate 5-phosphatase activity / negative regulation of monocyte differentiation ...inositol-polyphosphate 5-phosphatase / negative regulation of neutrophil differentiation / inositol-1,3,4,5-tetrakisphosphate 5-phosphatase activity / inositol-polyphosphate 5-phosphatase activity / phosphatidylinositol-3,4,5-trisphosphate 3-phosphatase activity / phosphatidylinositol-3,4,5-trisphosphate 5-phosphatase / phosphoinositide 5-phosphatase / phosphatidylinositol-3,4,5-trisphosphate 5-phosphatase activity / phosphatidylinositol-4,5-bisphosphate 5-phosphatase activity / negative regulation of monocyte differentiation / phosphatidylinositol dephosphorylation / phosphatidylinositol biosynthetic process / phosphate-containing compound metabolic process / negative regulation of natural killer cell mediated cytotoxicity / negative regulation of bone resorption / positive regulation of B cell differentiation / negative regulation of B cell proliferation / negative regulation of osteoclast differentiation / Synthesis of IP3 and IP4 in the cytosol / Synthesis of PIPs at the plasma membrane / PECAM1 interactions / immunoglobulin mediated immune response / negative regulation of interleukin-6 production / Interleukin receptor SHC signaling / regulation of immune response / negative regulation of signal transduction / positive regulation of erythrocyte differentiation / determination of adult lifespan / SH3 domain binding / Signaling by CSF1 (M-CSF) in myeloid cells / Downstream TCR signaling / T cell receptor signaling pathway / cytoskeleton / intracellular signal transduction / positive regulation of apoptotic process / membrane raft / apoptotic process / signal transduction / plasma membrane / cytosol / cytoplasm Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.45 Å MOLECULAR REPLACEMENT / Resolution: 1.45 Å | ||||||
 Authors Authors | Bradshaw, W.J. / Moreira, T. / Pascoa, T.C. / Bountra, C. / Chalk, R. / von Delft, F. / Brennan, P.E. / Gileadi, O. | ||||||
| Funding support |  United States, 1items United States, 1items
| ||||||
 Citation Citation |  Journal: Structure / Year: 2024 Journal: Structure / Year: 2024Title: Regulation of inositol 5-phosphatase activity by the C2 domain of SHIP1 and SHIP2. Authors: Bradshaw, W.J. / Kennedy, E.C. / Moreira, T. / Smith, L.A. / Chalk, R. / Katis, V.L. / Benesch, J.L.P. / Brennan, P.E. / Murphy, E.J. / Gileadi, O. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  8pdh.cif.gz 8pdh.cif.gz | 215 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb8pdh.ent.gz pdb8pdh.ent.gz | Display |  PDB format PDB format | |
| PDBx/mmJSON format |  8pdh.json.gz 8pdh.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/pd/8pdh https://data.pdbj.org/pub/pdb/validation_reports/pd/8pdh ftp://data.pdbj.org/pub/pdb/validation_reports/pd/8pdh ftp://data.pdbj.org/pub/pdb/validation_reports/pd/8pdh | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  5rw2C  5rw3C  5rw4C  5rw5C  5rw6C  5rw7C  5rw8C  5rw9C  5rwaC  5rwbC  5rwcC  5rwdC  5rweC  5rwfC  5rwgC  5rwhC  5rwiC  5rwjC  5rwkC  5rwlC  5rwmC  5rwnC  5rwoC  5rwpC  5rwqC  5rwrC  5rwsC  5rwtC  5rwuC  5rwvC  5rwwC  5rwxC  5rwyC  5rwzC  5rx0C  5rx1C  5rx2C  5rx3C  5rx4C  5rx5C  5rx6C  5rx7C  5rx8C  5rx9C  5rxaC  5rxbC  5rxcC  5rxdC  5rxeC  5rxfC  5rxgC  5rxhC  5rxiC  5rxjC  5rxkC  5rxlC  5rxmC  5rxoC  5rxpC  5rxqC  5rxrC  5rxsC  5rxtC  5rxuC  5rxvC  5rxwC  5rxxC  5rxyC  5rxzC  5ry0C  5ry1C  5ry2C  5ry3C  5ry4C  5ry5C  5ry6C  5ry7C  5ry8C  5ry9C  5ryaC  5rybC  5rycC  5rydC  5ryeC  5ryfC  5rygC  5ryhC  5ryiC  5ryjC  5rykC  5rylC  6ibdC  6xy7C  8pdgC  8pdiC  8pdjC C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 52877.574 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: INPP5D, SHIP, SHIP1 / Plasmid: pFB-HGT-LIC / Cell line (production host): Sf9 / Production host: Homo sapiens (human) / Gene: INPP5D, SHIP, SHIP1 / Plasmid: pFB-HGT-LIC / Cell line (production host): Sf9 / Production host:  References: UniProt: Q92835, phosphatidylinositol-3,4,5-trisphosphate 5-phosphatase, inositol-polyphosphate 5-phosphatase, phosphoinositide 5-phosphatase | ||||||
|---|---|---|---|---|---|---|---|
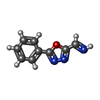
| #2: Chemical | ChemComp-YBU / ( | ||||||
| #3: Chemical | ChemComp-EDO / | ||||||
| #4: Chemical | | #5: Water | ChemComp-HOH / | Has ligand of interest | Y | Has protein modification | Y | |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.09 Å3/Da / Density % sol: 41.18 % |
|---|---|
| Crystal grow | Temperature: 293 K / Method: vapor diffusion, sitting drop / pH: 6.5 Details: 30 mM sodium nitrate, 30 mM dibasic sodium phosphate, 30 mM ammonium sulphate, 100 mM MES/imidazole, 20 % PEG 500 MME, 10% PEG 20,000 |
-Data collection
| Diffraction | Mean temperature: 100 K / Serial crystal experiment: N |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  Diamond Diamond  / Beamline: I03 / Wavelength: 0.9763 Å / Beamline: I03 / Wavelength: 0.9763 Å |
| Detector | Type: DECTRIS EIGER2 XE 16M / Detector: PIXEL / Date: Apr 24, 2021 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.9763 Å / Relative weight: 1 |
| Reflection | Resolution: 1.45→79.2 Å / Num. obs: 79287 / % possible obs: 100 % / Redundancy: 12.9 % / CC1/2: 0.998 / Net I/σ(I): 8 |
| Reflection shell | Resolution: 1.45→1.47 Å / Num. unique obs: 3864 / CC1/2: 0.453 / % possible all: 100 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT / Resolution: 1.45→51.276 Å / Cor.coef. Fo:Fc: 0.978 / Cor.coef. Fo:Fc free: 0.962 / SU B: 3.923 / SU ML: 0.062 / Cross valid method: FREE R-VALUE / ESU R: 0.071 / ESU R Free: 0.07 MOLECULAR REPLACEMENT / Resolution: 1.45→51.276 Å / Cor.coef. Fo:Fc: 0.978 / Cor.coef. Fo:Fc free: 0.962 / SU B: 3.923 / SU ML: 0.062 / Cross valid method: FREE R-VALUE / ESU R: 0.071 / ESU R Free: 0.07 Details: Hydrogens have been added in their riding positions
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.2 Å / Solvent model: MASK BULK SOLVENT | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 20.658 Å2
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.45→51.276 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
|
 Movie
Movie Controller
Controller


 PDBj
PDBj