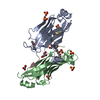
Entry Database : PDB / ID : 8iiyTitle Crystal structure of MBP fused GAS41 YEATS domain in complex with H3K14ac peptide Histone H3.1 Maltodextrin-binding protein,YEATS domain-containing protein 4 Keywords / / / / Function / homology Function Domain/homology Component
/ / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / Biological species Escherichia coli (E. coli)Homo sapiens (human)Method / / / Resolution : 2.15 Å Authors Kikuchi, M. / Umehara, T. Funding support Organization Grant number Country Japan Society for the Promotion of Science (JSPS)
Journal : Proc.Natl.Acad.Sci.USA / Year : 2023Title : GAS41 promotes H2A.Z deposition through recognition of the N terminus of histone H3 by the YEATS domain.Authors : Kikuchi, M. / Takase, S. / Konuma, T. / Noritsugu, K. / Sekine, S. / Ikegami, T. / Ito, A. / Umehara, T. History Deposition Feb 24, 2023 Deposition site / Processing site Revision 1.0 Nov 1, 2023 Provider / Type Revision 1.1 Nov 15, 2023 Group / Category / chem_comp_bond / Item / _chem_comp_bond.atom_id_2Revision 1.2 Nov 13, 2024 Group / Category / pdbx_modification_feature / Item
Show all Show less
 Yorodumi
Yorodumi Open data
Open data Basic information
Basic information Components
Components Keywords
Keywords Function and homology information
Function and homology information
 Homo sapiens (human)
Homo sapiens (human) X-RAY DIFFRACTION /
X-RAY DIFFRACTION /  SYNCHROTRON /
SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.15 Å
MOLECULAR REPLACEMENT / Resolution: 2.15 Å  Authors
Authors Japan, 1items
Japan, 1items  Citation
Citation Journal: Proc.Natl.Acad.Sci.USA / Year: 2023
Journal: Proc.Natl.Acad.Sci.USA / Year: 2023 Structure visualization
Structure visualization Molmil
Molmil Jmol/JSmol
Jmol/JSmol Downloads & links
Downloads & links Download
Download 8iiy.cif.gz
8iiy.cif.gz PDBx/mmCIF format
PDBx/mmCIF format pdb8iiy.ent.gz
pdb8iiy.ent.gz PDB format
PDB format 8iiy.json.gz
8iiy.json.gz PDBx/mmJSON format
PDBx/mmJSON format Other downloads
Other downloads https://data.pdbj.org/pub/pdb/validation_reports/ii/8iiy
https://data.pdbj.org/pub/pdb/validation_reports/ii/8iiy ftp://data.pdbj.org/pub/pdb/validation_reports/ii/8iiy
ftp://data.pdbj.org/pub/pdb/validation_reports/ii/8iiy

 F&H Search
F&H Search Links
Links Assembly
Assembly
 Components
Components
 Homo sapiens (human)
Homo sapiens (human)
 Homo sapiens (human) / References: UniProt: P68431
Homo sapiens (human) / References: UniProt: P68431 X-RAY DIFFRACTION / Number of used crystals: 1
X-RAY DIFFRACTION / Number of used crystals: 1  Sample preparation
Sample preparation SYNCHROTRON / Site:
SYNCHROTRON / Site:  SPring-8
SPring-8  / Beamline: BL26B2 / Wavelength: 1 Å
/ Beamline: BL26B2 / Wavelength: 1 Å Processing
Processing MOLECULAR REPLACEMENT / Resolution: 2.15→45 Å / Cor.coef. Fo:Fc: 0.971 / Cor.coef. Fo:Fc free: 0.94 / SU B: 14.555 / SU ML: 0.176 / Cross valid method: THROUGHOUT / ESU R Free: 0.193 / Stereochemistry target values: MAXIMUM LIKELIHOOD / Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS
MOLECULAR REPLACEMENT / Resolution: 2.15→45 Å / Cor.coef. Fo:Fc: 0.971 / Cor.coef. Fo:Fc free: 0.94 / SU B: 14.555 / SU ML: 0.176 / Cross valid method: THROUGHOUT / ESU R Free: 0.193 / Stereochemistry target values: MAXIMUM LIKELIHOOD / Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS Movie
Movie Controller
Controller


 PDBj
PDBj














