+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 8d59 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Crystal structure of human METTL1 in complex with SAM | ||||||||||||
 Components Components | tRNA (guanine-N(7)-)-methyltransferase | ||||||||||||
 Keywords Keywords | TRANSFERASE / Cancer protein | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationinternal mRNA (guanine-N7-)-methyltransferase activity / tRNA (m7G46) methyltransferase complex / tRNA (guanine-N7)-methylation / RNA (guanine-N7)-methylation / tRNA (guanine46-N7)-methyltransferase / tRNA (guanine(46)-N7)-methyltransferase activity / tRNA stabilization / tRNA methyltransferase complex / tRNA modification in the nucleus and cytosol / tRNA modification ...internal mRNA (guanine-N7-)-methyltransferase activity / tRNA (m7G46) methyltransferase complex / tRNA (guanine-N7)-methylation / RNA (guanine-N7)-methylation / tRNA (guanine46-N7)-methyltransferase / tRNA (guanine(46)-N7)-methyltransferase activity / tRNA stabilization / tRNA methyltransferase complex / tRNA modification in the nucleus and cytosol / tRNA modification / tRNA methylation / cellular response to stress / Transferases; Transferring one-carbon groups; Methyltransferases / tRNA binding / nucleolus / nucleoplasm / nucleus / cytosol Similarity search - Function | ||||||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.26 Å MOLECULAR REPLACEMENT / Resolution: 2.26 Å | ||||||||||||
 Authors Authors | Raj, R. / Babu, K. / Nam, Y. | ||||||||||||
| Funding support |  United States, 3items United States, 3items
| ||||||||||||
 Citation Citation |  Journal: Nature / Year: 2023 Journal: Nature / Year: 2023Title: Structures and mechanisms of tRNA methylation by METTL1-WDR4. Authors: Victor M Ruiz-Arroyo / Rishi Raj / Kesavan Babu / Otgonbileg Onolbaatar / Paul H Roberts / Yunsun Nam /  Abstract: Specific, regulated modification of RNAs is important for proper gene expression. tRNAs are rich with various chemical modifications that affect their stability and function. 7-Methylguanosine (mG) ...Specific, regulated modification of RNAs is important for proper gene expression. tRNAs are rich with various chemical modifications that affect their stability and function. 7-Methylguanosine (mG) at tRNA position 46 is a conserved modification that modulates steady-state tRNA levels to affect cell growth. The METTL1-WDR4 complex generates mG46 in humans, and dysregulation of METTL1-WDR4 has been linked to brain malformation and multiple cancers. Here we show how METTL1 and WDR4 cooperate to recognize RNA substrates and catalyse methylation. A crystal structure of METTL1-WDR4 and cryo-electron microscopy structures of METTL1-WDR4-tRNA show that the composite protein surface recognizes the tRNA elbow through shape complementarity. The cryo-electron microscopy structures of METTL1-WDR4-tRNA with S-adenosylmethionine or S-adenosylhomocysteine along with METTL1 crystal structures provide additional insights into the catalytic mechanism by revealing the active site in multiple states. The METTL1 N terminus couples cofactor binding with conformational changes in the tRNA, the catalytic loop and the WDR4 C terminus, acting as the switch to activate mG methylation. Thus, our structural models explain how post-translational modifications of the METTL1 N terminus can regulate methylation. Together, our work elucidates the core and regulatory mechanisms underlying mG modification by METTL1, providing the framework to understand its contribution to biology and disease. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  8d59.cif.gz 8d59.cif.gz | 70.5 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb8d59.ent.gz pdb8d59.ent.gz | 39.8 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  8d59.json.gz 8d59.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/d5/8d59 https://data.pdbj.org/pub/pdb/validation_reports/d5/8d59 ftp://data.pdbj.org/pub/pdb/validation_reports/d5/8d59 ftp://data.pdbj.org/pub/pdb/validation_reports/d5/8d59 | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  8d58C  8d5bC  8d9kC  8d9lC  8eg0C  3ckkS S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||||||
| Unit cell |
|
- Components
Components
-Protein , 1 types, 1 molecules A
| #1: Protein | Mass: 30412.713 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: METTL1, C12orf1 / Production host: Homo sapiens (human) / Gene: METTL1, C12orf1 / Production host:  References: UniProt: Q9UBP6, tRNA (guanine46-N7)-methyltransferase, Transferases; Transferring one-carbon groups; Methyltransferases |
|---|
-Non-polymers , 6 types, 59 molecules 


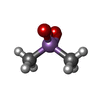







| #2: Chemical | ChemComp-SAM / | ||||||
|---|---|---|---|---|---|---|---|
| #3: Chemical | ChemComp-GOL / | ||||||
| #4: Chemical | | #5: Chemical | ChemComp-CAC / | #6: Chemical | ChemComp-CL / | #7: Water | ChemComp-HOH / | |
-Details
| Has ligand of interest | Y |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.46 Å3/Da / Density % sol: 50.03 % |
|---|---|
| Crystal grow | Temperature: 295.15 K / Method: vapor diffusion, hanging drop / pH: 5.7 Details: 50 mM Sodium cacodylate pH 5.7, 1.5 M Lithium sulfate, 10 mM Magnesium sulfate |
-Data collection
| Diffraction | Mean temperature: 100 K / Serial crystal experiment: N |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  APS APS  / Beamline: 19-ID / Wavelength: 0.9789 Å / Beamline: 19-ID / Wavelength: 0.9789 Å |
| Detector | Type: DECTRIS PILATUS3 6M / Detector: PIXEL / Date: Dec 2, 2020 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.9789 Å / Relative weight: 1 |
| Reflection | Resolution: 2.25→37.25 Å / Num. obs: 12936 / % possible obs: 99.2 % / Redundancy: 15.5 % / Biso Wilson estimate: 47.02 Å2 / CC1/2: 0.983 / Rmerge(I) obs: 0.292 / Rpim(I) all: 0.07 / Net I/σ(I): 13.5 |
| Reflection shell | Resolution: 2.25→2.29 Å / Redundancy: 5.7 % / Rmerge(I) obs: 1.89 / Mean I/σ(I) obs: 1.2 / Num. unique obs: 579 / CC1/2: 0.414 / Rpim(I) all: 0.797 / % possible all: 91.6 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 3CKK Resolution: 2.26→37.25 Å / SU ML: 0.2414 / Cross valid method: FREE R-VALUE / σ(F): 1.37 / Phase error: 28.0713 Stereochemistry target values: GeoStd + Monomer Library + CDL v1.2
| |||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.1 Å / Solvent model: FLAT BULK SOLVENT MODEL | |||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 53.48 Å2 | |||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.26→37.25 Å
| |||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||
| LS refinement shell |
|
 Movie
Movie Controller
Controller






 PDBj
PDBj











