[English] 日本語
 Yorodumi
Yorodumi- PDB-8d3f: Crystal structure of human STAT1 in complex with the repeat regio... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 8d3f | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal structure of human STAT1 in complex with the repeat region from Toxoplasma protein TgIST | ||||||
 Components Components | Signal transducer and activator of transcription 1-alpha/beta,Inhibitor of STAT1-dependent transcription TgIST | ||||||
 Keywords Keywords | TRANSCRIPTION / Signal transduction / Transcriptional activation / Pathogen effector / Interferon signaling | ||||||
| Function / homology |  Function and homology information Function and homology informationmetanephric mesenchymal cell differentiation / negative regulation of metanephric nephron tubule epithelial cell differentiation / negative regulation by virus of viral protein levels in host cell / renal tubule development / ISGF3 complex / response to interferon-beta / metanephric mesenchymal cell proliferation involved in metanephros development / interleukin-27-mediated signaling pathway / negative regulation of mesenchymal to epithelial transition involved in metanephros morphogenesis / CCR5 chemokine receptor binding ...metanephric mesenchymal cell differentiation / negative regulation of metanephric nephron tubule epithelial cell differentiation / negative regulation by virus of viral protein levels in host cell / renal tubule development / ISGF3 complex / response to interferon-beta / metanephric mesenchymal cell proliferation involved in metanephros development / interleukin-27-mediated signaling pathway / negative regulation of mesenchymal to epithelial transition involved in metanephros morphogenesis / CCR5 chemokine receptor binding / Interleukin-9 signaling / Interleukin-21 signaling / interleukin-7-mediated signaling pathway / interleukin-9-mediated signaling pathway / Signaling by cytosolic FGFR1 fusion mutants / tumor necrosis factor receptor binding / Interleukin-27 signaling / Interleukin-35 Signalling / cell surface receptor signaling pathway via STAT / positive regulation of mesenchymal cell proliferation / blood circulation / NOTCH3 Intracellular Domain Regulates Transcription / Interleukin-20 family signaling / Interleukin-6 signaling / type I interferon-mediated signaling pathway / histone acetyltransferase binding / negative regulation of endothelial cell proliferation / ubiquitin-like protein ligase binding / Regulation of IFNA/IFNB signaling / positive regulation of interferon-alpha production / cell surface receptor signaling pathway via JAK-STAT / response to cAMP / type II interferon-mediated signaling pathway / Growth hormone receptor signaling / response to type II interferon / response to mechanical stimulus / Regulation of IFNG signaling / RNA polymerase II core promoter sequence-specific DNA binding / Signaling by PDGFRA transmembrane, juxtamembrane and kinase domain mutants / Signaling by PDGFRA extracellular domain mutants / Signaling by CSF3 (G-CSF) / cellular response to interferon-beta / positive regulation of defense response to virus by host / response to cytokine / response to nutrient / positive regulation of smooth muscle cell proliferation / Downstream signal transduction / positive regulation of erythrocyte differentiation / protein phosphatase 2A binding / Signaling by phosphorylated juxtamembrane, extracellular and kinase domain KIT mutants / negative regulation of angiogenesis / negative regulation of canonical NF-kappaB signal transduction / Turbulent (oscillatory, disturbed) flow shear stress activates signaling by PIEZO1 and integrins in endothelial cells / transcription corepressor binding / tumor necrosis factor-mediated signaling pathway / RNA polymerase II transcription regulatory region sequence-specific DNA binding / promoter-specific chromatin binding / response to hydrogen peroxide / Downregulation of SMAD2/3:SMAD4 transcriptional activity / defense response / Signaling by SCF-KIT / PKR-mediated signaling / Inactivation of CSF3 (G-CSF) signaling / response to peptide hormone / cellular response to type II interferon / RNA polymerase II transcription regulator complex / ISG15 antiviral mechanism / transcription coactivator binding / cellular response to insulin stimulus / Interferon gamma signaling / positive regulation of nitric oxide biosynthetic process / Signaling by CSF1 (M-CSF) in myeloid cells / Regulation of RUNX2 expression and activity / Interferon alpha/beta signaling / Signaling by ALK fusions and activated point mutants / regulation of cell population proliferation / double-stranded DNA binding / Interleukin-4 and Interleukin-13 signaling / regulation of apoptotic process / histone binding / defense response to virus / DNA-binding transcription factor activity, RNA polymerase II-specific / cadherin binding / RNA polymerase II cis-regulatory region sequence-specific DNA binding / response to xenobiotic stimulus / DNA-binding transcription factor activity / axon / dendrite / regulation of transcription by RNA polymerase II / positive regulation of DNA-templated transcription / chromatin / nucleolus / perinuclear region of cytoplasm / SARS-CoV-2 activates/modulates innate and adaptive immune responses / enzyme binding / negative regulation of transcription by RNA polymerase II / protein homodimerization activity / positive regulation of transcription by RNA polymerase II / protein-containing complex / nucleoplasm Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.97 Å MOLECULAR REPLACEMENT / Resolution: 2.97 Å | ||||||
 Authors Authors | Huang, Z. / Liu, H. / Nix, J.C. / Amarasinghe, G.K. / Sibley, L.D. | ||||||
| Funding support |  United States, 1items United States, 1items
| ||||||
 Citation Citation |  Journal: Nat Commun / Year: 2022 Journal: Nat Commun / Year: 2022Title: The intrinsically disordered protein TgIST from Toxoplasma gondii inhibits STAT1 signaling by blocking cofactor recruitment. Authors: Huang, Z. / Liu, H. / Nix, J. / Xu, R. / Knoverek, C.R. / Bowman, G.R. / Amarasinghe, G.K. / Sibley, L.D. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  8d3f.cif.gz 8d3f.cif.gz | 153.4 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb8d3f.ent.gz pdb8d3f.ent.gz | 95.1 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  8d3f.json.gz 8d3f.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/d3/8d3f https://data.pdbj.org/pub/pdb/validation_reports/d3/8d3f ftp://data.pdbj.org/pub/pdb/validation_reports/d3/8d3f ftp://data.pdbj.org/pub/pdb/validation_reports/d3/8d3f | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  1bf5S S: Starting model for refinement |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 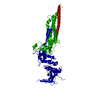
| ||||||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 73359.930 Da / Num. of mol.: 1 / Fragment: STAT1-binding region Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human), (gene. exp.) Homo sapiens (human), (gene. exp.)  Gene: STAT1, TGRH88_087790 / Production host:  |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 3.8 Å3/Da / Density % sol: 67.62 % |
|---|---|
| Crystal grow | Temperature: 293 K / Method: vapor diffusion, hanging drop Details: 0.1 M Bis-Tris pH 6.3-6.0, 3 M sodium chloride and 50 mM sodium citrate tribasic PH range: 6.0-6.3 |
-Data collection
| Diffraction | Mean temperature: 100 K / Serial crystal experiment: N |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  ALS ALS  / Beamline: 4.2.2 / Wavelength: 1.0722 Å / Beamline: 4.2.2 / Wavelength: 1.0722 Å |
| Detector | Type: RDI CMOS_8M / Detector: CMOS / Date: Apr 18, 2021 |
| Radiation | Monochromator: M / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1.0722 Å / Relative weight: 1 |
| Reflection | Resolution: 2.97→46.64 Å / Num. obs: 23640 / % possible obs: 100 % / Redundancy: 29 % / Biso Wilson estimate: 79.63 Å2 / CC1/2: 0.999 / Rmerge(I) obs: 0.176 / Rpim(I) all: 0.033 / Rrim(I) all: 0.179 / Rsym value: 0.176 / Net I/σ(I): 20.3 |
| Reflection shell | Resolution: 2.97→3.15 Å / Redundancy: 28.4 % / Rmerge(I) obs: 1.961 / Mean I/σ(I) obs: 1.9 / Num. unique obs: 3764 / CC1/2: 0.874 / Rpim(I) all: 0.372 / Rrim(I) all: 1.997 / % possible all: 100 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 1BF5 Resolution: 2.97→46.64 Å / SU ML: 0.3952 / Cross valid method: FREE R-VALUE / σ(F): 1.35 / Phase error: 36.8527 Stereochemistry target values: GeoStd + Monomer Library + CDL v1.2
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å / Solvent model: FLAT BULK SOLVENT MODEL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 95.33 Å2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.97→46.64 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
|
 Movie
Movie Controller
Controller


 PDBj
PDBj












