[English] 日本語
 Yorodumi
Yorodumi- PDB-8ctg: Extracellular architecture of an engineered canonical Wnt signali... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 8ctg | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Extracellular architecture of an engineered canonical Wnt signaling ternary complex | ||||||||||||
 Components Components |
| ||||||||||||
 Keywords Keywords | SIGNALING PROTEIN / signaling complex / beta-catenin | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationnegative regulation of cardiac cell fate specification / Spemann organizer formation / neural crest cell fate commitment / Wnt-Frizzled-LRP5/6 complex / Regulation of FZD by ubiquitination / Negative regulation of TCF-dependent signaling by WNT ligand antagonists / Asymmetric localization of PCP proteins / neural crest formation / Signaling by RNF43 mutants / head development ...negative regulation of cardiac cell fate specification / Spemann organizer formation / neural crest cell fate commitment / Wnt-Frizzled-LRP5/6 complex / Regulation of FZD by ubiquitination / Negative regulation of TCF-dependent signaling by WNT ligand antagonists / Asymmetric localization of PCP proteins / neural crest formation / Signaling by RNF43 mutants / head development / embryonic axis specification / kinase inhibitor activity / toxin transmembrane transporter activity / Wnt receptor activity / low-density lipoprotein particle receptor activity / Wnt-protein binding / cellular response to cholesterol / midbrain dopaminergic neuron differentiation / dopaminergic neuron differentiation / frizzled binding / Wnt signalosome / anterior/posterior axis specification / Disassembly of the destruction complex and recruitment of AXIN to the membrane / neural crest cell differentiation / mesoderm development / negative regulation of smooth muscle cell apoptotic process / cell fate commitment / protein serine/threonine kinase inhibitor activity / canonical Wnt signaling pathway / neuronal dense core vesicle / coreceptor activity / positive regulation of cell cycle / Regulation of FZD by ubiquitination / cytokine activity / protein localization to plasma membrane / PDZ domain binding / TCF dependent signaling in response to WNT / cell-cell adhesion / response to peptide hormone / G protein-coupled receptor activity / endocytosis / Wnt signaling pathway / neuron differentiation / T cell differentiation in thymus / nervous system development / : / positive regulation of cytosolic calcium ion concentration / protease binding / cytoplasmic vesicle / early endosome membrane / angiogenesis / chemical synaptic transmission / membrane raft / signaling receptor binding / neuronal cell body / ubiquitin protein ligase binding / synapse / positive regulation of DNA-templated transcription / cell surface / endoplasmic reticulum / Golgi apparatus / protein homodimerization activity / positive regulation of transcription by RNA polymerase II / extracellular space / extracellular region / identical protein binding / plasma membrane Similarity search - Function | ||||||||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | ||||||||||||
| Method | ELECTRON MICROSCOPY / single particle reconstruction / cryo EM / Resolution: 3.8 Å | ||||||||||||
 Authors Authors | Tsutsumi, N. / Jude, K.M. / Garcia, K.C. | ||||||||||||
| Funding support |  United States, 3items United States, 3items
| ||||||||||||
 Citation Citation |  Journal: Proc Natl Acad Sci U S A / Year: 2023 Journal: Proc Natl Acad Sci U S A / Year: 2023Title: Structure of the Wnt-Frizzled-LRP6 initiation complex reveals the basis for coreceptor discrimination. Authors: Naotaka Tsutsumi / Sunhee Hwang / Deepa Waghray / Simon Hansen / Kevin M Jude / Nan Wang / Yi Miao / Caleb R Glassman / Nathanael A Caveney / Claudia Y Janda / Rami N Hannoush / K Christopher Garcia /    Abstract: Wnt morphogens are critical for embryonic development and tissue regeneration. Canonical Wnts form ternary receptor complexes composed of tissue-specific Frizzled (Fzd) receptors together with the ...Wnt morphogens are critical for embryonic development and tissue regeneration. Canonical Wnts form ternary receptor complexes composed of tissue-specific Frizzled (Fzd) receptors together with the shared LRP5/6 coreceptors to initiate β-catenin signaling. The cryo-EM structure of a ternary initiation complex of an affinity-matured XWnt8-Frizzled8-LRP6 complex elucidates the basis of coreceptor discrimination by canonical Wnts by means of their N termini and linker domains that engage the LRP6 E1E2 domain funnels. Chimeric Wnts bearing modular linker "grafts" were able to transfer LRP6 domain specificity between different Wnts and enable non-canonical Wnt5a to signal through the canonical pathway. Synthetic peptides comprising the linker domain serve as Wnt-specific antagonists. The structure of the ternary complex provides a topological blueprint for the orientation and proximity of Frizzled and LRP6 within the Wnt cell surface signalosome. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  8ctg.cif.gz 8ctg.cif.gz | 163.6 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb8ctg.ent.gz pdb8ctg.ent.gz | 106.7 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  8ctg.json.gz 8ctg.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  8ctg_validation.pdf.gz 8ctg_validation.pdf.gz | 1.5 MB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  8ctg_full_validation.pdf.gz 8ctg_full_validation.pdf.gz | 1.5 MB | Display | |
| Data in XML |  8ctg_validation.xml.gz 8ctg_validation.xml.gz | 42.4 KB | Display | |
| Data in CIF |  8ctg_validation.cif.gz 8ctg_validation.cif.gz | 63.6 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/ct/8ctg https://data.pdbj.org/pub/pdb/validation_reports/ct/8ctg ftp://data.pdbj.org/pub/pdb/validation_reports/ct/8ctg ftp://data.pdbj.org/pub/pdb/validation_reports/ct/8ctg | HTTPS FTP |
-Related structure data
| Related structure data |  26989MC  8ffeC M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
| #1: Protein | Mass: 15061.343 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   |
|---|---|
| #2: Protein | Mass: 36676.590 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  |
| #3: Protein | Mass: 73384.648 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: LRP6 / Production host: Homo sapiens (human) / Gene: LRP6 / Production host:  |
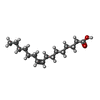
| #4: Chemical | ChemComp-PAM / |
| Has ligand of interest | N |
| Has protein modification | Y |
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method: single particle reconstruction |
- Sample preparation
Sample preparation
| Component |
| |||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular weight | Value: 0.13 MDa / Experimental value: NO | |||||||||||||||||||||||||||||||||||
| Source (natural) |
| |||||||||||||||||||||||||||||||||||
| Source (recombinant) |
| |||||||||||||||||||||||||||||||||||
| Buffer solution | pH: 8 | |||||||||||||||||||||||||||||||||||
| Buffer component |
| |||||||||||||||||||||||||||||||||||
| Specimen | Conc.: 0.5 mg/ml / Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: YES / Details: haXWnt8-mFzd8CRD-hLRP6E1E2 | |||||||||||||||||||||||||||||||||||
| Specimen support | Grid material: GOLD / Grid mesh size: 300 divisions/in. / Grid type: UltrAuFoil R1.2/1.3 | |||||||||||||||||||||||||||||||||||
| Vitrification | Instrument: FEI VITROBOT MARK IV / Cryogen name: ETHANE / Humidity: 100 % / Chamber temperature: 277 K / Details: 3 s blotting before plunging |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TITAN KRIOS |
| Electron gun | Electron source:  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD / Nominal magnification: 29000 X / Calibrated magnification: 58680 X / Nominal defocus max: 2000 nm / Nominal defocus min: 1000 nm / Cs: 2.7 mm / C2 aperture diameter: 100 µm / Alignment procedure: COMA FREE |
| Specimen holder | Cryogen: NITROGEN / Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER |
| Image recording | Electron dose: 55 e/Å2 / Detector mode: SUPER-RESOLUTION / Film or detector model: GATAN K3 (6k x 4k) / Num. of grids imaged: 1 / Num. of real images: 10077 |
- Processing
Processing
| Software | Name: PHENIX / Version: 1.20.1_4487: / Classification: refinement | ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EM software |
| ||||||||||||||||||||||||||||||||
| CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION | ||||||||||||||||||||||||||||||||
| Particle selection | Num. of particles selected: 3840364 / Details: Particles include duplicates and junk. | ||||||||||||||||||||||||||||||||
| Symmetry | Point symmetry: C1 (asymmetric) | ||||||||||||||||||||||||||||||||
| 3D reconstruction | Resolution: 3.8 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 82235 Details: Model resolution is substantially lower and estimated to be ~10 angstrom based on the map-model FSC. Symmetry type: POINT | ||||||||||||||||||||||||||||||||
| Atomic model building | Protocol: FLEXIBLE FIT / Space: REAL | ||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller


 PDBj
PDBj