+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 8crs | |||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | CryoEM Structure of nitrogenase MoFe-protein in detergent | |||||||||||||||||||||||||||||||||||||||
 Components Components | (Nitrogenase molybdenum-iron protein ...) x 2 | |||||||||||||||||||||||||||||||||||||||
 Keywords Keywords | METAL BINDING PROTEIN / OXIDOREDUCTASE / Nitrogenase / metalloenzyme | |||||||||||||||||||||||||||||||||||||||
| Function / homology |  Function and homology information Function and homology informationmolybdenum-iron nitrogenase complex / nitrogenase / nitrogenase activity / nitrogen fixation / iron-sulfur cluster binding / ATP binding / metal ion binding Similarity search - Function | |||||||||||||||||||||||||||||||||||||||
| Biological species |  Azotobacter vinelandii (bacteria) Azotobacter vinelandii (bacteria) | |||||||||||||||||||||||||||||||||||||||
| Method | ELECTRON MICROSCOPY / single particle reconstruction / cryo EM / Resolution: 2.04 Å | |||||||||||||||||||||||||||||||||||||||
 Authors Authors | Warmack, R.A. / Rees, D.C. | |||||||||||||||||||||||||||||||||||||||
| Funding support |  United States, 3items United States, 3items
| |||||||||||||||||||||||||||||||||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2023 Journal: Nat Commun / Year: 2023Title: Structural consequences of turnover-induced homocitrate loss in nitrogenase. Authors: Rebeccah A Warmack / Ailiena O Maggiolo / Andres Orta / Belinda B Wenke / James B Howard / Douglas C Rees /  Abstract: Nitrogenase catalyzes the ATP-dependent reduction of dinitrogen to ammonia during the process of biological nitrogen fixation that is essential for sustaining life. The active site FeMo-cofactor ...Nitrogenase catalyzes the ATP-dependent reduction of dinitrogen to ammonia during the process of biological nitrogen fixation that is essential for sustaining life. The active site FeMo-cofactor contains a [7Fe:1Mo:9S:1C] metallocluster coordinated with an R-homocitrate (HCA) molecule. Here, we establish through single particle cryoEM and chemical analysis of two forms of the Azotobacter vinelandii MoFe-protein - a high pH turnover inactivated species and a ∆NifV variant that cannot synthesize HCA - that loss of HCA is coupled to α-subunit domain and FeMo-cofactor disordering, and formation of a histidine coordination site. We further find a population of the ∆NifV variant complexed to an endogenous protein identified through structural and proteomic approaches as the uncharacterized protein NafT. Recognition by endogenous NafT demonstrates the physiological relevance of the HCA-compromised form, perhaps for cofactor insertion or repair. Our results point towards a dynamic active site in which HCA plays a role in enabling nitrogenase catalysis by facilitating activation of the FeMo-cofactor from a relatively stable form to a state capable of reducing dinitrogen under ambient conditions. | |||||||||||||||||||||||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  8crs.cif.gz 8crs.cif.gz | 447.2 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb8crs.ent.gz pdb8crs.ent.gz | 356.2 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  8crs.json.gz 8crs.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/cr/8crs https://data.pdbj.org/pub/pdb/validation_reports/cr/8crs ftp://data.pdbj.org/pub/pdb/validation_reports/cr/8crs ftp://data.pdbj.org/pub/pdb/validation_reports/cr/8crs | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  26957MC  8dbxC  8enlC  8enmC  8ennC  8enoC M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
-Nitrogenase molybdenum-iron protein ... , 2 types, 4 molecules ACBD
| #1: Protein | Mass: 54289.906 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)  Azotobacter vinelandii (bacteria) / References: UniProt: P07328, nitrogenase Azotobacter vinelandii (bacteria) / References: UniProt: P07328, nitrogenase#2: Protein | Mass: 59404.684 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)  Azotobacter vinelandii (bacteria) / References: UniProt: P07329, nitrogenase Azotobacter vinelandii (bacteria) / References: UniProt: P07329, nitrogenase |
|---|
-Non-polymers , 6 types, 1704 molecules 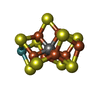









| #3: Chemical | | #4: Chemical | #5: Chemical | ChemComp-1N7 / #6: Chemical | #7: Chemical | #8: Water | ChemComp-HOH / | |
|---|
-Details
| Has ligand of interest | N |
|---|---|
| Has protein modification | N |
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method: single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: Heterotetrameric nitrogenase MoFe-protein / Type: COMPLEX / Entity ID: #1-#2 / Source: NATURAL |
|---|---|
| Source (natural) | Organism:  Azotobacter vinelandii (bacteria) Azotobacter vinelandii (bacteria) |
| Buffer solution | pH: 7.8 |
| Specimen | Conc.: 1.5 mg/ml / Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: YES |
| Vitrification | Cryogen name: ETHANE-PROPANE |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: TFS KRIOS |
| Electron gun | Electron source:  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: OTHER FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: OTHER |
| Electron lens | Mode: BRIGHT FIELD / Nominal defocus max: -3000 nm / Nominal defocus min: -800 nm |
| Image recording | Electron dose: 60 e/Å2 / Film or detector model: GATAN K3 (6k x 4k) |
- Processing
Processing
| Software | Name: PHENIX / Version: 1.20.1_4487: / Classification: refinement | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EM software | Name: PHENIX / Category: model refinement | ||||||||||||||||||||||||
| CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION | ||||||||||||||||||||||||
| 3D reconstruction | Resolution: 2.04 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 137629 / Symmetry type: POINT | ||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller








 PDBj
PDBj





