+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 7ybx | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Crystal structure of FGFR4(V550M) kinase domain with 10z | ||||||||||||
 Components Components | Fibroblast growth factor receptor 4 | ||||||||||||
 Keywords Keywords | TRANSFERASE/INHIBITOR / Kinase / Inhibitor / STRUCTURAL PROTEIN / TRANSFERASE-TRANSFERASE INHIBITOR complex / TRANSFERASE / TRANSFERASE-INHIBITOR complex | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationFGFR4 mutant receptor activation / betaKlotho-mediated ligand binding / regulation of extracellular matrix disassembly / positive regulation of catalytic activity / phosphate ion homeostasis / regulation of bile acid biosynthetic process / FGFR4 ligand binding and activation / Phospholipase C-mediated cascade; FGFR4 / fibroblast growth factor receptor activity / positive regulation of DNA biosynthetic process ...FGFR4 mutant receptor activation / betaKlotho-mediated ligand binding / regulation of extracellular matrix disassembly / positive regulation of catalytic activity / phosphate ion homeostasis / regulation of bile acid biosynthetic process / FGFR4 ligand binding and activation / Phospholipase C-mediated cascade; FGFR4 / fibroblast growth factor receptor activity / positive regulation of DNA biosynthetic process / PI-3K cascade:FGFR4 / fibroblast growth factor binding / regulation of lipid metabolic process / positive regulation of proteolysis / PI3K Cascade / fibroblast growth factor receptor signaling pathway / SHC-mediated cascade:FGFR4 / Signaling by FGFR4 in disease / transport vesicle / FRS-mediated FGFR4 signaling / peptidyl-tyrosine phosphorylation / cholesterol homeostasis / Negative regulation of FGFR4 signaling / receptor protein-tyrosine kinase / Constitutive Signaling by Aberrant PI3K in Cancer / cell migration / glucose homeostasis / PIP3 activates AKT signaling / heparin binding / protein autophosphorylation / PI5P, PP2A and IER3 Regulate PI3K/AKT Signaling / RAF/MAP kinase cascade / positive regulation of ERK1 and ERK2 cascade / receptor complex / endosome / positive regulation of cell population proliferation / positive regulation of gene expression / endoplasmic reticulum / Golgi apparatus / extracellular region / ATP binding / plasma membrane Similarity search - Function | ||||||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  MOLECULAR REPLACEMENT / Resolution: 2.233 Å MOLECULAR REPLACEMENT / Resolution: 2.233 Å | ||||||||||||
 Authors Authors | Chen, X.J. / Lin, Q.M. / Chen, Y.H. | ||||||||||||
| Funding support |  China, 3items China, 3items
| ||||||||||||
 Citation Citation |  Journal: J.Med.Chem. / Year: 2022 Journal: J.Med.Chem. / Year: 2022Title: Design, Synthesis, and Biological Evaluation of 5-Formyl-pyrrolo[3,2- b ]pyridine-3-carboxamides as New Selective, Potent, and Reversible-Covalent FGFR4 Inhibitors. Authors: Yang, F. / Chen, X. / Song, X. / Ortega, R. / Lin, X. / Deng, W. / Guo, J. / Tu, Z. / Patterson, A.V. / Smaill, J.B. / Chen, Y. / Lu, X. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  7ybx.cif.gz 7ybx.cif.gz | 80.4 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb7ybx.ent.gz pdb7ybx.ent.gz | 57 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  7ybx.json.gz 7ybx.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/yb/7ybx https://data.pdbj.org/pub/pdb/validation_reports/yb/7ybx ftp://data.pdbj.org/pub/pdb/validation_reports/yb/7ybx ftp://data.pdbj.org/pub/pdb/validation_reports/yb/7ybx | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  7yboC  7ybpC  7yc1C  7yc3C  7wctS S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 34540.895 Da / Num. of mol.: 1 / Fragment: kinase domain / Mutation: V550M,R664E Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: FGFR4, JTK2, TKF / Production host: Homo sapiens (human) / Gene: FGFR4, JTK2, TKF / Production host:  References: UniProt: P22455, receptor protein-tyrosine kinase | ||||||
|---|---|---|---|---|---|---|---|
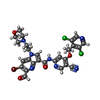
| #2: Chemical | ChemComp-IH7 / ~{ | ||||||
| #3: Chemical | ChemComp-SO4 / #4: Water | ChemComp-HOH / | Has ligand of interest | Y | Has protein modification | Y | |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.24 Å3/Da / Density % sol: 45.03 % |
|---|---|
| Crystal grow | Temperature: 277 K / Method: vapor diffusion, hanging drop / pH: 4.5 Details: 0.1 M Bis-Tris (pH 4.5), 0.2 M Li2SO4, 16-19 % PEG 3350 |
-Data collection
| Diffraction | Mean temperature: 100 K / Serial crystal experiment: N |
|---|---|
| Diffraction source | Source:  ROTATING ANODE / Type: Cu FINE FOCUS / Wavelength: 1.587 Å ROTATING ANODE / Type: Cu FINE FOCUS / Wavelength: 1.587 Å |
| Detector | Type: RIGAKU / Detector: IMAGE PLATE / Date: May 5, 2022 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1.587 Å / Relative weight: 1 |
| Reflection | Resolution: 2.233→42.823 Å / Num. obs: 14877 / % possible obs: 99.67 % / Redundancy: 7.3 % / Biso Wilson estimate: 22.4 Å2 / CC1/2: 0.98 / Rrim(I) all: 0.2216 / Net I/σ(I): 9.75 |
| Reflection shell | Resolution: 2.233→2.313 Å / Redundancy: 6.6 % / Mean I/σ(I) obs: 6.08 / Num. unique obs: 1436 / CC1/2: 0.934 / % possible all: 96.7 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 7WCT Resolution: 2.233→42.823 Å / SU ML: 0.21 / Cross valid method: THROUGHOUT / σ(F): 1.45 / Phase error: 21.72 / Stereochemistry target values: ML
| ||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å / Solvent model: FLAT BULK SOLVENT MODEL | ||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso max: 82.81 Å2 / Biso mean: 27.0086 Å2 / Biso min: 8.39 Å2 | ||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: final / Resolution: 2.233→42.823 Å
| ||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||
| LS refinement shell | Refine-ID: X-RAY DIFFRACTION / Rfactor Rfree error: 0
|
 Movie
Movie Controller
Controller



 PDBj
PDBj