[English] 日本語
 Yorodumi
Yorodumi- PDB-7wc4: Crystal structure of serotonin 2A receptor in complex with serotonin -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 7wc4 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Crystal structure of serotonin 2A receptor in complex with serotonin | ||||||||||||
 Components Components | 5-hydroxytryptamine receptor 2A,5-hydroxytryptamine receptor 2A,5-hydroxytryptamine receptor 2A,Soluble cytochrome b562 | ||||||||||||
 Keywords Keywords | MEMBRANE PROTEIN / serotonin 2A receptor / serotonin / 5-HT | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationprotein localization to cytoskeleton / positive regulation of heat generation / 1-(4-iodo-2,5-dimethoxyphenyl)propan-2-amine binding / Gq/11-coupled serotonin receptor activity / positive regulation of phosphatidylinositol biosynthetic process / G protein-coupled serotonin receptor signaling pathway / G protein-coupled serotonin receptor complex / neurofilament / cell body fiber / phospholipase C-activating serotonin receptor signaling pathway ...protein localization to cytoskeleton / positive regulation of heat generation / 1-(4-iodo-2,5-dimethoxyphenyl)propan-2-amine binding / Gq/11-coupled serotonin receptor activity / positive regulation of phosphatidylinositol biosynthetic process / G protein-coupled serotonin receptor signaling pathway / G protein-coupled serotonin receptor complex / neurofilament / cell body fiber / phospholipase C-activating serotonin receptor signaling pathway / artery smooth muscle contraction / positive regulation of cytokine production involved in immune response / serotonin receptor activity / Serotonin receptors / G protein-coupled serotonin receptor activity / serotonin receptor signaling pathway / sensitization / urinary bladder smooth muscle contraction / neurotransmitter receptor activity / serotonin binding / positive regulation of platelet aggregation / negative regulation of synaptic transmission, glutamatergic / positive regulation of DNA biosynthetic process / temperature homeostasis / detection of temperature stimulus involved in sensory perception of pain / regulation of dopamine secretion / negative regulation of potassium ion transport / protein tyrosine kinase activator activity / positive regulation of vasoconstriction / positive regulation of execution phase of apoptosis / detection of mechanical stimulus involved in sensory perception of pain / G protein-coupled receptor signaling pathway, coupled to cyclic nucleotide second messenger / positive regulation of fat cell differentiation / behavioral response to cocaine / release of sequestered calcium ion into cytosol / presynaptic modulation of chemical synaptic transmission / positive regulation of glycolytic process / dendritic shaft / glycolytic process / caveola / memory / intracellular calcium ion homeostasis / positive regulation of inflammatory response / positive regulation of neuron apoptotic process / positive regulation of cytosolic calcium ion concentration / virus receptor activity / presynaptic membrane / cytoplasmic vesicle / G alpha (q) signalling events / chemical synaptic transmission / postsynaptic membrane / positive regulation of ERK1 and ERK2 cascade / response to xenobiotic stimulus / axon / neuronal cell body / positive regulation of cell population proliferation / dendrite / protein-containing complex binding / glutamatergic synapse / identical protein binding / plasma membrane Similarity search - Function | ||||||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 3.2 Å MOLECULAR REPLACEMENT / Resolution: 3.2 Å | ||||||||||||
 Authors Authors | Cao, D. / Yu, J. / Wang, H. / Luo, Z. / Liu, X. / He, L. / Qi, J. / Fan, L. / Tang, L. / Chen, Z. ...Cao, D. / Yu, J. / Wang, H. / Luo, Z. / Liu, X. / He, L. / Qi, J. / Fan, L. / Tang, L. / Chen, Z. / Li, J. / Cheng, J. / Wang, S. | ||||||||||||
| Funding support |  China, 3items China, 3items
| ||||||||||||
 Citation Citation |  Journal: Science / Year: 2022 Journal: Science / Year: 2022Title: Structure-based discovery of nonhallucinogenic psychedelic analogs. Authors: Cao, D. / Yu, J. / Wang, H. / Luo, Z. / Liu, X. / He, L. / Qi, J. / Fan, L. / Tang, L. / Chen, Z. / Li, J. / Cheng, J. / Wang, S. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  7wc4.cif.gz 7wc4.cif.gz | 93.7 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb7wc4.ent.gz pdb7wc4.ent.gz | 67.2 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  7wc4.json.gz 7wc4.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/wc/7wc4 https://data.pdbj.org/pub/pdb/validation_reports/wc/7wc4 ftp://data.pdbj.org/pub/pdb/validation_reports/wc/7wc4 ftp://data.pdbj.org/pub/pdb/validation_reports/wc/7wc4 | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  7wc5C  7wc6C  7wc7C  7wc8C  7wc9C  6a93S S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
| ||||||||
| Components on special symmetry positions |
|
- Components
Components
-Protein , 1 types, 1 molecules A
| #1: Protein | Mass: 42056.258 Da / Num. of mol.: 1 / Mutation: S162K,M164W,M1007W,R1098I,H1102I,R1106G,S372N Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: HTR2A, HTR2, HTR2A, cybC / Production host: Homo sapiens (human) / Gene: HTR2A, HTR2, HTR2A, cybC / Production host:  |
|---|
-Non-polymers , 6 types, 10 molecules 



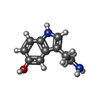






| #2: Chemical | | #3: Chemical | ChemComp-1PE / | #4: Chemical | #5: Chemical | ChemComp-MG / | #6: Chemical | ChemComp-SRO / | #7: Water | ChemComp-HOH / | |
|---|
-Details
| Has ligand of interest | Y |
|---|---|
| Has protein modification | Y |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.89 Å3/Da / Density % sol: 57.44 % |
|---|---|
| Crystal grow | Temperature: 293.15 K / Method: lipidic cubic phase Details: 100 mM Tris-HCl , 100 mM sodium acetate trihydrate, 30% (v/v) polyethylene glycol 400 (PEG400), 4% (v/v) Polypropylene glycol P 400 PH range: 7.0-7.5 |
-Data collection
| Diffraction | Mean temperature: 100 K / Serial crystal experiment: N |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  SPring-8 SPring-8  / Beamline: BL45XU / Wavelength: 1 Å / Beamline: BL45XU / Wavelength: 1 Å |
| Detector | Type: DECTRIS PILATUS 6M / Detector: PIXEL / Date: Oct 21, 2020 |
| Radiation | Monochromator: M / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1 Å / Relative weight: 1 |
| Reflection | Resolution: 3.2→48.05 Å / Num. obs: 8511 / % possible obs: 99 % / Redundancy: 9.1 % / CC1/2: 0.996 / Net I/σ(I): 5.2 |
| Reflection shell | Resolution: 3.2→3.31 Å / Redundancy: 6.4 % / Mean I/σ(I) obs: 1 / Num. unique obs: 1456 / CC1/2: 0.323 / % possible all: 96.6 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 6A93 Resolution: 3.2→48.05 Å / Cor.coef. Fo:Fc: 0.912 / Cor.coef. Fo:Fc free: 0.898 / SU B: 31.711 / SU ML: 0.502 / Cross valid method: THROUGHOUT / σ(F): 0 / ESU R Free: 0.518 / Stereochemistry target values: MAXIMUM LIKELIHOOD Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS U VALUES : REFINED INDIVIDUALLY
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.2 Å / Solvent model: MASK | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso max: 274.5 Å2 / Biso mean: 71.165 Å2 / Biso min: 30.31 Å2
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: final / Resolution: 3.2→48.05 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 3.2→3.283 Å / Total num. of bins used: 20
|
 Movie
Movie Controller
Controller












 PDBj
PDBj
















