[English] 日本語
 Yorodumi
Yorodumi- PDB-7u1s: Cryo-EM structure of the pancreatic ATP-sensitive potassium chann... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 7u1s | ||||||
|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of the pancreatic ATP-sensitive potassium channel bound to ATP and repaglinide with SUR1-out conformation | ||||||
 Components Components |
| ||||||
 Keywords Keywords | MEMBRANE PROTEIN / KATP channel / SUR1 / Kir6.2 / repaglinide / RPG / sulfonylurea receptor / potassium transport | ||||||
| Function / homology |  Function and homology information Function and homology informationRegulation of insulin secretion / ATP sensitive Potassium channels / ABC-family proteins mediated transport / ATP-activated inward rectifier potassium channel activity / response to resveratrol / inward rectifying potassium channel / sulfonylurea receptor activity / ventricular cardiac muscle tissue development / cell body fiber / CAMKK-AMPK signaling cascade ...Regulation of insulin secretion / ATP sensitive Potassium channels / ABC-family proteins mediated transport / ATP-activated inward rectifier potassium channel activity / response to resveratrol / inward rectifying potassium channel / sulfonylurea receptor activity / ventricular cardiac muscle tissue development / cell body fiber / CAMKK-AMPK signaling cascade / voltage-gated monoatomic ion channel activity involved in regulation of presynaptic membrane potential / regulation of monoatomic ion transmembrane transport / ATPase-coupled monoatomic cation transmembrane transporter activity / inward rectifier potassium channel activity / nervous system process / : / ankyrin binding / Ion homeostasis / response to ATP / response to stress / response to testosterone / potassium ion import across plasma membrane / action potential / intercalated disc / axolemma / voltage-gated potassium channel activity / potassium channel activity / ABC-type transporter activity / cellular response to nutrient levels / heat shock protein binding / potassium ion transmembrane transport / T-tubule / regulation of insulin secretion / acrosomal vesicle / response to ischemia / determination of adult lifespan / regulation of membrane potential / positive regulation of protein localization to plasma membrane / cellular response to glucose stimulus / negative regulation of insulin secretion / sarcolemma / potassium ion transport / cellular response to nicotine / glucose metabolic process / cellular response to tumor necrosis factor / nuclear envelope / response to estradiol / presynaptic membrane / transmembrane transporter binding / response to hypoxia / endosome / response to xenobiotic stimulus / neuronal cell body / apoptotic process / glutamatergic synapse / protein-containing complex / ATP hydrolysis activity / ATP binding / metal ion binding / plasma membrane / cytoplasm Similarity search - Function | ||||||
| Biological species |   Cricetus cricetus (black-bellied hamster) Cricetus cricetus (black-bellied hamster) | ||||||
| Method | ELECTRON MICROSCOPY / single particle reconstruction / cryo EM / Resolution: 3.8 Å | ||||||
 Authors Authors | Shyng, S.L. / Sung, M.W. / Driggers, C.M. | ||||||
| Funding support |  United States, 1items United States, 1items
| ||||||
 Citation Citation |  Journal: J Mol Biol / Year: 2022 Journal: J Mol Biol / Year: 2022Title: Ligand-mediated Structural Dynamics of a Mammalian Pancreatic K Channel. Authors: Min Woo Sung / Camden M Driggers / Barmak Mostofian / John D Russo / Bruce L Patton / Daniel M Zuckerman / Show-Ling Shyng /  Abstract: Regulation of pancreatic K channels involves orchestrated interactions of their subunits, Kir6.2 and SUR1, and ligands. Previously we reported K channel cryo-EM structures in the presence and absence ...Regulation of pancreatic K channels involves orchestrated interactions of their subunits, Kir6.2 and SUR1, and ligands. Previously we reported K channel cryo-EM structures in the presence and absence of pharmacological inhibitors and ATP, focusing on the mechanisms by which inhibitors act as pharmacological chaperones of K channels (Martin et al., 2019). Here we analyzed the same cryo-EM datasets with a focus on channel conformational dynamics to elucidate structural correlates pertinent to ligand interactions and channel gating. We found pharmacological inhibitors and ATP enrich a channel conformation in which the Kir6.2 cytoplasmic domain is closely associated with the transmembrane domain, while depleting one where the Kir6.2 cytoplasmic domain is extended away into the cytoplasm. This conformational change remodels a network of intra- and inter-subunit interactions as well as the ATP and PIP binding pockets. The structures resolved key contacts between the distal N-terminus of Kir6.2 and SUR1's ABC module involving residues implicated in channel function and showed a SUR1 residue, K134, participates in PIP binding. Molecular dynamics simulations revealed two Kir6.2 residues, K39 and R54, that mediate both ATP and PIP binding, suggesting a mechanism for competitive gating by ATP and PIP. #1:  Journal: Elife / Year: 2019 Journal: Elife / Year: 2019Title: Mechanism of pharmacochaperoning in a mammalian K channel revealed by cryo-EM. Authors: Gregory M Martin / Min Woo Sung / Zhongying Yang / Laura M Innes / Balamurugan Kandasamy / Larry L David / Craig Yoshioka / Show-Ling Shyng /  Abstract: ATP-sensitive potassium (K) channels composed of a pore-forming Kir6.2 potassium channel and a regulatory ABC transporter sulfonylurea receptor 1 (SUR1) regulate insulin secretion in pancreatic β- ...ATP-sensitive potassium (K) channels composed of a pore-forming Kir6.2 potassium channel and a regulatory ABC transporter sulfonylurea receptor 1 (SUR1) regulate insulin secretion in pancreatic β-cells to maintain glucose homeostasis. Mutations that impair channel folding or assembly prevent cell surface expression and cause congenital hyperinsulinism. Structurally diverse K inhibitors are known to act as pharmacochaperones to correct mutant channel expression, but the mechanism is unknown. Here, we compare cryoEM structures of a mammalian K channel bound to pharmacochaperones glibenclamide, repaglinide, and carbamazepine. We found all three drugs bind within a common pocket in SUR1. Further, we found the N-terminus of Kir6.2 inserted within the central cavity of the SUR1 ABC core, adjacent the drug binding pocket. The findings reveal a common mechanism by which diverse compounds stabilize the Kir6.2 N-terminus within SUR1's ABC core, allowing it to act as a firm 'handle' for the assembly of metastable mutant SUR1-Kir6.2 complexes. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  7u1s.cif.gz 7u1s.cif.gz | 590.1 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb7u1s.ent.gz pdb7u1s.ent.gz | 391.9 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  7u1s.json.gz 7u1s.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  7u1s_validation.pdf.gz 7u1s_validation.pdf.gz | 1.7 MB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  7u1s_full_validation.pdf.gz 7u1s_full_validation.pdf.gz | 1.7 MB | Display | |
| Data in XML |  7u1s_validation.xml.gz 7u1s_validation.xml.gz | 81.1 KB | Display | |
| Data in CIF |  7u1s_validation.cif.gz 7u1s_validation.cif.gz | 116.6 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/u1/7u1s https://data.pdbj.org/pub/pdb/validation_reports/u1/7u1s ftp://data.pdbj.org/pub/pdb/validation_reports/u1/7u1s ftp://data.pdbj.org/pub/pdb/validation_reports/u1/7u1s | HTTPS FTP |
-Related structure data
| Related structure data |  26304MC  7tysC  7tytC  7u1eC  7u1qC  7u24C  7u2xC  7u6yC  7u7mC  7uaaC  7uqrC C: citing same article ( M: map data used to model this data |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
-Protein , 2 types, 5 molecules ABCDE
| #1: Protein | Mass: 43661.762 Da / Num. of mol.: 4 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   #2: Protein | | Mass: 177333.578 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Cricetus cricetus (black-bellied hamster) Cricetus cricetus (black-bellied hamster)Gene: ABCC8, SUR / Cell line (production host): INS - 1 CELLS CLONE 832/13 / Production host:  |
|---|
-Sugars , 1 types, 1 molecules
| #3: Polysaccharide | 2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose Source method: isolated from a genetically manipulated source |
|---|
-Non-polymers , 6 types, 24 molecules 




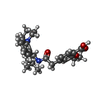





| #4: Chemical | ChemComp-ATP / #5: Chemical | ChemComp-K / | #6: Chemical | ChemComp-POV / ( #7: Chemical | ChemComp-P5S / #8: Chemical | ChemComp-PTY / #9: Chemical | ChemComp-BJX / | |
|---|
-Details
| Has ligand of interest | Y |
|---|---|
| Has protein modification | Y |
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method: single particle reconstruction |
- Sample preparation
Sample preparation
| Component |
| ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Source (natural) |
| ||||||||||||||||||||||||||||
| Source (recombinant) |
| ||||||||||||||||||||||||||||
| Buffer solution | pH: 7.5 | ||||||||||||||||||||||||||||
| Specimen | Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: YES | ||||||||||||||||||||||||||||
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TITAN KRIOS |
| Electron gun | Electron source:  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD / Nominal defocus max: 2600 nm / Nominal defocus min: 1000 nm / Cs: 2.7 mm |
| Image recording | Electron dose: 40 e/Å2 / Film or detector model: FEI FALCON III (4k x 4k) |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EM software |
| ||||||||||||||||||||||||||||||
| CTF correction | Type: NONE | ||||||||||||||||||||||||||||||
| 3D reconstruction | Resolution: 3.8 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 28289 / Symmetry type: POINT | ||||||||||||||||||||||||||||||
| Atomic model building | Space: REAL | ||||||||||||||||||||||||||||||
| Atomic model building | 3D fitting-ID: 1 / Source name: PDB / Type: experimental model
| ||||||||||||||||||||||||||||||
| Refinement | Cross valid method: NONE Stereochemistry target values: GeoStd + Monomer Library + CDL v1.2 | ||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 56.68 Å2 | ||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller











 PDBj
PDBj











