[English] 日本語
 Yorodumi
Yorodumi- PDB-7sqo: Structure of the orexin-2 receptor(OX2R) bound to TAK-925, Gi and... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 7sqo | ||||||
|---|---|---|---|---|---|---|---|
| Title | Structure of the orexin-2 receptor(OX2R) bound to TAK-925, Gi and scFv16 | ||||||
 Components Components |
| ||||||
 Keywords Keywords | MEMBRANE PROTEIN / CLASS A GPCR | ||||||
| Function / homology |  Function and homology information Function and homology informationregulation of circadian sleep/wake cycle, wakefulness / circadian sleep/wake cycle process / orexin receptor activity / Orexin and neuropeptides FF and QRFP bind to their respective receptors / Olfactory Signaling Pathway / Sensory perception of sweet, bitter, and umami (glutamate) taste / Synthesis, secretion, and inactivation of Glucagon-like Peptide-1 (GLP-1) / neuropeptide receptor activity / Activation of the phototransduction cascade / feeding behavior ...regulation of circadian sleep/wake cycle, wakefulness / circadian sleep/wake cycle process / orexin receptor activity / Orexin and neuropeptides FF and QRFP bind to their respective receptors / Olfactory Signaling Pathway / Sensory perception of sweet, bitter, and umami (glutamate) taste / Synthesis, secretion, and inactivation of Glucagon-like Peptide-1 (GLP-1) / neuropeptide receptor activity / Activation of the phototransduction cascade / feeding behavior / locomotion / Activation of G protein gated Potassium channels / G-protein activation / G beta:gamma signalling through PI3Kgamma / Prostacyclin signalling through prostacyclin receptor / G beta:gamma signalling through PLC beta / ADP signalling through P2Y purinoceptor 1 / Thromboxane signalling through TP receptor / Presynaptic function of Kainate receptors / G beta:gamma signalling through CDC42 / Inhibition of voltage gated Ca2+ channels via Gbeta/gamma subunits / G alpha (12/13) signalling events / Glucagon-type ligand receptors / G beta:gamma signalling through BTK / ADP signalling through P2Y purinoceptor 12 / Adrenaline,noradrenaline inhibits insulin secretion / Cooperation of PDCL (PhLP1) and TRiC/CCT in G-protein beta folding / Ca2+ pathway / Thrombin signalling through proteinase activated receptors (PARs) / G alpha (z) signalling events / Extra-nuclear estrogen signaling / G alpha (s) signalling events / G alpha (q) signalling events / G alpha (i) signalling events / Glucagon-like Peptide-1 (GLP1) regulates insulin secretion / High laminar flow shear stress activates signaling by PIEZO1 and PECAM1:CDH5:KDR in endothelial cells / Vasopressin regulates renal water homeostasis via Aquaporins / peptide hormone binding / regulation of cytosolic calcium ion concentration / neuropeptide signaling pathway / cellular response to hormone stimulus / adenylate cyclase inhibitor activity / positive regulation of protein localization to cell cortex / T cell migration / Adenylate cyclase inhibitory pathway / D2 dopamine receptor binding / response to prostaglandin E / adenylate cyclase regulator activity / G protein-coupled serotonin receptor binding / adenylate cyclase-inhibiting serotonin receptor signaling pathway / cellular response to forskolin / regulation of mitotic spindle organization / Regulation of insulin secretion / positive regulation of cholesterol biosynthetic process / negative regulation of insulin secretion / G protein-coupled receptor binding / response to peptide hormone / adenylate cyclase-inhibiting G protein-coupled receptor signaling pathway / adenylate cyclase-modulating G protein-coupled receptor signaling pathway / centriolar satellite / G-protein beta/gamma-subunit complex binding / ADP signalling through P2Y purinoceptor 12 / photoreceptor disc membrane / Adrenaline,noradrenaline inhibits insulin secretion / GDP binding / G alpha (z) signalling events / cellular response to catecholamine stimulus / ADORA2B mediated anti-inflammatory cytokines production / adenylate cyclase-activating dopamine receptor signaling pathway / GPER1 signaling / G-protein beta-subunit binding / cellular response to prostaglandin E stimulus / heterotrimeric G-protein complex / sensory perception of taste / signaling receptor complex adaptor activity / G protein activity / retina development in camera-type eye / GTPase binding / midbody / cell cortex / G alpha (i) signalling events / G alpha (s) signalling events / phospholipase C-activating G protein-coupled receptor signaling pathway / G alpha (q) signalling events / chemical synaptic transmission / Hydrolases; Acting on acid anhydrides; Acting on GTP to facilitate cellular and subcellular movement / Extra-nuclear estrogen signaling / cell population proliferation / ciliary basal body / G protein-coupled receptor signaling pathway / lysosomal membrane / cell division / GTPase activity / synapse / centrosome / GTP binding / protein-containing complex binding / nucleolus / magnesium ion binding / Golgi apparatus Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) synthetic construct (others) | ||||||
| Method | ELECTRON MICROSCOPY / single particle reconstruction / cryo EM / Resolution: 3.17 Å | ||||||
 Authors Authors | McGrath, A.P. / Kang, Y. / Flinspach, M. | ||||||
| Funding support | 1items
| ||||||
 Citation Citation |  Journal: Nat Commun / Year: 2022 Journal: Nat Commun / Year: 2022Title: Molecular mechanism of the wake-promoting agent TAK-925. Authors: Jie Yin / Yanyong Kang / Aaron P McGrath / Karen Chapman / Megan Sjodt / Eiji Kimura / Atsutoshi Okabe / Tatsuki Koike / Yuhei Miyanohana / Yuji Shimizu / Rameshu Rallabandi / Peng Lian / ...Authors: Jie Yin / Yanyong Kang / Aaron P McGrath / Karen Chapman / Megan Sjodt / Eiji Kimura / Atsutoshi Okabe / Tatsuki Koike / Yuhei Miyanohana / Yuji Shimizu / Rameshu Rallabandi / Peng Lian / Xiaochen Bai / Mack Flinspach / Jef K De Brabander / Daniel M Rosenbaum /    Abstract: The OX orexin receptor (OXR) is a highly expressed G protein-coupled receptor (GPCR) in the brain that regulates wakefulness and circadian rhythms in humans. Antagonism of OXR is a proven therapeutic ...The OX orexin receptor (OXR) is a highly expressed G protein-coupled receptor (GPCR) in the brain that regulates wakefulness and circadian rhythms in humans. Antagonism of OXR is a proven therapeutic strategy for insomnia drugs, and agonism of OXR is a potentially powerful approach for narcolepsy type 1, which is characterized by the death of orexinergic neurons. Until recently, agonism of OXR had been considered 'undruggable.' We harness cryo-electron microscopy of OXR-G protein complexes to determine how the first clinically tested OXR agonist TAK-925 can activate OXR in a highly selective manner. Two structures of TAK-925-bound OXR with either a G mimetic or G reveal that TAK-925 binds at the same site occupied by antagonists, yet interacts with the transmembrane helices to trigger activating microswitches. Our structural and mutagenesis data show that TAK-925's selectivity is mediated by subtle differences between OX and OX receptor subtypes at the orthosteric pocket. Finally, differences in the polarity of interactions at the G protein binding interfaces help to rationalize OXR's coupling selectivity for G signaling. The mechanisms of TAK-925's binding, activation, and selectivity presented herein will aid in understanding the efficacy of small molecule OXR agonists for narcolepsy and other circadian disorders. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  7sqo.cif.gz 7sqo.cif.gz | 380.1 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb7sqo.ent.gz pdb7sqo.ent.gz | 300 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  7sqo.json.gz 7sqo.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/sq/7sqo https://data.pdbj.org/pub/pdb/validation_reports/sq/7sqo ftp://data.pdbj.org/pub/pdb/validation_reports/sq/7sqo ftp://data.pdbj.org/pub/pdb/validation_reports/sq/7sqo | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  25389MC  7sr8C M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
-Guanine nucleotide-binding protein ... , 3 types, 3 molecules ABG
| #2: Protein | Mass: 40459.039 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: GNAI1 / Production host: Homo sapiens (human) / Gene: GNAI1 / Production host:  Trichoplusia ni (cabbage looper) / References: UniProt: P63096 Trichoplusia ni (cabbage looper) / References: UniProt: P63096 |
|---|---|
| #3: Protein | Mass: 39151.855 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Trichoplusia ni (cabbage looper) / References: UniProt: P62871 Trichoplusia ni (cabbage looper) / References: UniProt: P62871 |
| #4: Protein | Mass: 7563.750 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Trichoplusia ni (cabbage looper) / References: UniProt: P63212 Trichoplusia ni (cabbage looper) / References: UniProt: P63212 |
-Protein / Antibody , 2 types, 2 molecules RE
| #1: Protein | Mass: 50733.289 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: HCRTR2 / Production host: Homo sapiens (human) / Gene: HCRTR2 / Production host:  Spodoptera (butterflies/moths) / References: UniProt: O43614 Spodoptera (butterflies/moths) / References: UniProt: O43614 |
|---|---|
| #5: Antibody | Mass: 26277.299 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.) synthetic construct (others) / Production host:  Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper) |
-Non-polymers , 2 types, 5 molecules 
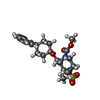

| #6: Chemical | ChemComp-OLA / #7: Chemical | ChemComp-A6F / | |
|---|
-Details
| Has ligand of interest | Y |
|---|---|
| Has protein modification | Y |
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method: single particle reconstruction |
- Sample preparation
Sample preparation
| Component |
| ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular weight | Experimental value: NO | ||||||||||||||||||||||||||||||||||||
| Source (natural) |
| ||||||||||||||||||||||||||||||||||||
| Source (recombinant) |
| ||||||||||||||||||||||||||||||||||||
| Buffer solution | pH: 7.2 | ||||||||||||||||||||||||||||||||||||
| Specimen | Conc.: 5 mg/ml / Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: YES | ||||||||||||||||||||||||||||||||||||
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TITAN KRIOS |
| Electron gun | Electron source:  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD / Nominal magnification: 130000 X / Cs: 2.7 mm |
| Image recording | Electron dose: 43.7 e/Å2 / Film or detector model: GATAN K2 SUMMIT (4k x 4k) Details: Cryo-EM movie stacks were collected on a Titan Krios microscope operated at 300 kV under EFTEM mode. Nanoprobe with 1um illumination area was used. Data were recorded on a postGIF Gatan K2 ...Details: Cryo-EM movie stacks were collected on a Titan Krios microscope operated at 300 kV under EFTEM mode. Nanoprobe with 1um illumination area was used. Data were recorded on a postGIF Gatan K2 summit camera at a nominal magnification of 130,000, using super-resolution counting model. Bioquantum energy filter was operated in the zero-energy-loss mode with an energy slit width of 20 eV. |
| EM imaging optics | Energyfilter name: GIF Bioquantum |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EM software |
| ||||||||||||||||||||||||
| Image processing | Details: Frames 2-30 were used during dose-weighted movie frame alignment to reduce effects of beam-induced motion. Parameters of the FEI Titan Krios contrast transfer function were subsequently ...Details: Frames 2-30 were used during dose-weighted movie frame alignment to reduce effects of beam-induced motion. Parameters of the FEI Titan Krios contrast transfer function were subsequently estimated for each micrograph. A total 647,261 particles were auto-picked using Template Picker and extracted in 220 x 220 pixels box using cryoSPARC. All particles were subject to three rounds of reference-free 2D classification. Four initial model were made using cryoSPARC based on selected particles. The final 3D refinement in cryoSPARC2 used 251,169 particles, producing a reconstruction with 3.17A nominal resolution. | ||||||||||||||||||||||||
| CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION | ||||||||||||||||||||||||
| Particle selection | Num. of particles selected: 647261 | ||||||||||||||||||||||||
| 3D reconstruction | Resolution: 3.17 Å / Resolution method: FSC 0.5 CUT-OFF / Num. of particles: 251169 / Symmetry type: POINT | ||||||||||||||||||||||||
| Atomic model building | Protocol: RIGID BODY FIT | ||||||||||||||||||||||||
| Refinement | Cross valid method: NONE Stereochemistry target values: GeoStd + Monomer Library + CDL v1.2 | ||||||||||||||||||||||||
| Displacement parameters | Biso mean: 107.86 Å2 | ||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller



 PDBj
PDBj
























