+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 7qll | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | rsKiiro Thermal annealing at 290K of 200K Cis intermediate | |||||||||
 Components Components | rsKiiro | |||||||||
 Keywords Keywords | FLUORESCENT PROTEIN / rsKiiro | |||||||||
| Function / homology | Green fluorescent protein, GFP / Green fluorescent protein-related / Green fluorescent protein / Green fluorescent protein / bioluminescence / generation of precursor metabolites and energy / Green to red photoconvertible GFP-like protein EosFP Function and homology information Function and homology information | |||||||||
| Biological species |  Lobophyllia hemprichii (invertebrata) Lobophyllia hemprichii (invertebrata) | |||||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.324 Å MOLECULAR REPLACEMENT / Resolution: 1.324 Å | |||||||||
 Authors Authors | van Thor, J.J. / Baxter, J.M. | |||||||||
| Funding support |  United Kingdom, 1items United Kingdom, 1items
| |||||||||
 Citation Citation |  Journal: Nat.Chem. / Year: 2023 Journal: Nat.Chem. / Year: 2023Title: Optical control of ultrafast structural dynamics in a fluorescent protein. Authors: Hutchison, C.D.M. / Baxter, J.M. / Fitzpatrick, A. / Dorlhiac, G. / Fadini, A. / Perrett, S. / Maghlaoui, K. / Lefevre, S.B. / Cordon-Preciado, V. / Ferreira, J.L. / Chukhutsina, V.U. / ...Authors: Hutchison, C.D.M. / Baxter, J.M. / Fitzpatrick, A. / Dorlhiac, G. / Fadini, A. / Perrett, S. / Maghlaoui, K. / Lefevre, S.B. / Cordon-Preciado, V. / Ferreira, J.L. / Chukhutsina, V.U. / Garratt, D. / Barnard, J. / Galinis, G. / Glencross, F. / Morgan, R.M. / Stockton, S. / Taylor, B. / Yuan, L. / Romei, M.G. / Lin, C.Y. / Marangos, J.P. / Schmidt, M. / Chatrchyan, V. / Buckup, T. / Morozov, D. / Park, J. / Park, S. / Eom, I. / Kim, M. / Jang, D. / Choi, H. / Hyun, H. / Park, G. / Nango, E. / Tanaka, R. / Owada, S. / Tono, K. / DePonte, D.P. / Carbajo, S. / Seaberg, M. / Aquila, A. / Boutet, S. / Barty, A. / Iwata, S. / Boxer, S.G. / Groenhof, G. / van Thor, J.J. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  7qll.cif.gz 7qll.cif.gz | 112.5 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb7qll.ent.gz pdb7qll.ent.gz | 82.9 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  7qll.json.gz 7qll.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/ql/7qll https://data.pdbj.org/pub/pdb/validation_reports/ql/7qll ftp://data.pdbj.org/pub/pdb/validation_reports/ql/7qll ftp://data.pdbj.org/pub/pdb/validation_reports/ql/7qll | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  7qliC  7qljC  7qlkC  7qlmC  7qlnC 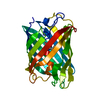 7qloC  5oq9S S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 25152.490 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Lobophyllia hemprichii (invertebrata) / Production host: Lobophyllia hemprichii (invertebrata) / Production host:  |
|---|---|
| #2: Chemical | ChemComp-GOL / |
| #3: Chemical | ChemComp-SO4 / |
| #4: Water | ChemComp-HOH / |
| Has ligand of interest | Y |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.22 Å3/Da / Density % sol: 44.65 % |
|---|---|
| Crystal grow | Temperature: 293 K / Method: batch mode / pH: 8.4 Details: 0.2 M LITHIUM SULFATE 0.1 M TRIS-CL PH 8.5 23-30% PEG 3350, |
-Data collection
| Diffraction | Mean temperature: 100 K / Serial crystal experiment: N |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  Diamond Diamond  / Beamline: I03 / Wavelength: 0.72932 Å / Beamline: I03 / Wavelength: 0.72932 Å |
| Detector | Type: DECTRIS PILATUS3 6M / Detector: PIXEL / Date: Dec 4, 2018 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.72932 Å / Relative weight: 1 |
| Reflection | Resolution: 1.324→53.703 Å / Num. obs: 44562 / % possible obs: 86.3 % / Redundancy: 15.7 % / CC1/2: 0.999 / Rmerge(I) obs: 0.087 / Rpim(I) all: 0.026 / Net I/σ(I): 15.7 |
| Reflection shell | Resolution: 1.336→1.4 Å / Mean I/σ(I) obs: 1.2 / Num. unique obs: 2224 / CC1/2: 0.455 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 5OQ9 Resolution: 1.324→53.703 Å / Cor.coef. Fo:Fc: 0.968 / Cor.coef. Fo:Fc free: 0.959 / WRfactor Rfree: 0.206 / WRfactor Rwork: 0.173 / SU B: 1.011 / SU ML: 0.039 / Average fsc free: 0.9265 / Average fsc work: 0.9333 / Cross valid method: FREE R-VALUE / ESU R: 0.06 / ESU R Free: 0.063 Details: Hydrogens have been added in their riding positions
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.2 Å / Solvent model: MASK BULK SOLVENT | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 16.881 Å2
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.324→53.703 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
|
 Movie
Movie Controller
Controller



 PDBj
PDBj






