[English] 日本語
 Yorodumi
Yorodumi- PDB-7pti: STRUCTURAL EFFECTS INDUCED BY REMOVAL OF A DISULFIDE BRIDGE. THE ... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 7pti | ||||||
|---|---|---|---|---|---|---|---|
| Title | STRUCTURAL EFFECTS INDUCED BY REMOVAL OF A DISULFIDE BRIDGE. THE X-RAY STRUCTURE OF THE C30A(SLASH)C51A MUTANT OF BASIC PANCREATIC TRYPSIN INHIBITOR AT 1.6 ANGSTROMS | ||||||
 Components Components | BOVINE PANCREATIC TRYPSIN INHIBITOR | ||||||
 Keywords Keywords | PROTEINASE INHIBITOR (TRYPSIN) | ||||||
| Function / homology |  Function and homology information Function and homology informationsulfate binding / negative regulation of platelet aggregation / potassium channel inhibitor activity / zymogen binding / molecular function inhibitor activity / negative regulation of thrombin-activated receptor signaling pathway / serine protease inhibitor complex / serine-type endopeptidase inhibitor activity / protease binding / calcium ion binding / extracellular space Similarity search - Function | ||||||
| Biological species |  | ||||||
| Method |  X-RAY DIFFRACTION / Resolution: 1.6 Å X-RAY DIFFRACTION / Resolution: 1.6 Å | ||||||
 Authors Authors | Eigenbrot, C. / Randal, M. / Kossiakoff, A.A. | ||||||
 Citation Citation |  Journal: Protein Eng. / Year: 1990 Journal: Protein Eng. / Year: 1990Title: Structural effects induced by removal of a disulfide-bridge: the X-ray structure of the C30A/C51A mutant of basic pancreatic trypsin inhibitor at 1.6 A. Authors: Eigenbrot, C. / Randal, M. / Kossiakoff, A.A. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  7pti.cif.gz 7pti.cif.gz | 25 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb7pti.ent.gz pdb7pti.ent.gz | 15.5 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  7pti.json.gz 7pti.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/pt/7pti https://data.pdbj.org/pub/pdb/validation_reports/pt/7pti ftp://data.pdbj.org/pub/pdb/validation_reports/pt/7pti ftp://data.pdbj.org/pub/pdb/validation_reports/pt/7pti | HTTPS FTP |
|---|
-Related structure data
| Similar structure data |
|---|
- Links
Links
- Assembly
Assembly
| Deposited unit | 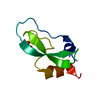
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 6463.438 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  |
|---|---|
| #2: Chemical | ChemComp-PO4 / |
| #3: Water | ChemComp-HOH / |
| Has protein modification | Y |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION X-RAY DIFFRACTION |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 1.93 Å3/Da / Density % sol: 36.3 % | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystal grow | *PLUS pH: 10 / Method: vapor diffusion, hanging drop | ||||||||||||||||||||
| Components of the solutions | *PLUS
|
-Data collection
| Reflection | *PLUS Highest resolution: 1.6 Å / Lowest resolution: 8 Å / Num. all: 6979 / % possible obs: 78 % / Observed criterion σ(F): 2 / Num. measured all: 5510 / Rmerge(I) obs: 0.146 |
|---|
- Processing
Processing
| Software | Name: PROLSQ / Classification: refinement | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Resolution: 1.6→8 Å / σ(I): 2 /
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.6→8 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement | *PLUS Highest resolution: 1.6 Å / Lowest resolution: 8 Å / Num. reflection obs: 5510 / σ(I): 2 / Rfactor obs: 0.17 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | *PLUS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | *PLUS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | *PLUS Total num. of bins used: 6 |
 Movie
Movie Controller
Controller










 PDBj
PDBj






