[English] 日本語
 Yorodumi
Yorodumi- PDB-7nwi: Mammalian pre-termination 80S ribosome with Empty-A site bound by... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 7nwi | ||||||
|---|---|---|---|---|---|---|---|
| Title | Mammalian pre-termination 80S ribosome with Empty-A site bound by Blasticidin S | ||||||
 Components Components |
| ||||||
 Keywords Keywords | RIBOSOME / Inhibitor 80S Termination Complex Blasticidin S Translation NMD | ||||||
| Function / homology |  Function and homology information Function and homology informationFormation of the ternary complex, and subsequently, the 43S complex / Formation of a pool of free 40S subunits / SRP-dependent cotranslational protein targeting to membrane / Major pathway of rRNA processing in the nucleolus and cytosol / Nonsense Mediated Decay (NMD) independent of the Exon Junction Complex (EJC) / Nonsense Mediated Decay (NMD) enhanced by the Exon Junction Complex (EJC) / Translation initiation complex formation / Ribosomal scanning and start codon recognition / L13a-mediated translational silencing of Ceruloplasmin expression / GTP hydrolysis and joining of the 60S ribosomal subunit ...Formation of the ternary complex, and subsequently, the 43S complex / Formation of a pool of free 40S subunits / SRP-dependent cotranslational protein targeting to membrane / Major pathway of rRNA processing in the nucleolus and cytosol / Nonsense Mediated Decay (NMD) independent of the Exon Junction Complex (EJC) / Nonsense Mediated Decay (NMD) enhanced by the Exon Junction Complex (EJC) / Translation initiation complex formation / Ribosomal scanning and start codon recognition / L13a-mediated translational silencing of Ceruloplasmin expression / GTP hydrolysis and joining of the 60S ribosomal subunit / Major pathway of rRNA processing in the nucleolus and cytosol / GTP hydrolysis and joining of the 60S ribosomal subunit / L13a-mediated translational silencing of Ceruloplasmin expression / SRP-dependent cotranslational protein targeting to membrane / Formation of a pool of free 40S subunits / Nonsense Mediated Decay (NMD) independent of the Exon Junction Complex (EJC) / Nonsense Mediated Decay (NMD) enhanced by the Exon Junction Complex (EJC) / ribosomal subunit / laminin receptor activity / ubiquitin ligase inhibitor activity / positive regulation of signal transduction by p53 class mediator / 90S preribosome / phagocytic cup / laminin binding / rough endoplasmic reticulum / translation regulator activity / ribosomal small subunit export from nucleus / gastrulation / MDM2/MDM4 family protein binding / cytosolic ribosome / class I DNA-(apurinic or apyrimidinic site) endonuclease activity / DNA-(apurinic or apyrimidinic site) lyase / ribosomal large subunit biogenesis / maturation of LSU-rRNA from tricistronic rRNA transcript (SSU-rRNA, 5.8S rRNA, LSU-rRNA) / positive regulation of apoptotic signaling pathway / maturation of SSU-rRNA / small-subunit processome / mRNA 5'-UTR binding / spindle / cytoplasmic ribonucleoprotein granule / rRNA processing / antimicrobial humoral immune response mediated by antimicrobial peptide / positive regulation of canonical Wnt signaling pathway / rhythmic process / heparin binding / regulation of translation / large ribosomal subunit / ribosome binding / virus receptor activity / ribosomal small subunit biogenesis / ribosomal small subunit assembly / 5S rRNA binding / ribosomal large subunit assembly / small ribosomal subunit / small ribosomal subunit rRNA binding / large ribosomal subunit rRNA binding / cytosolic small ribosomal subunit / defense response to Gram-negative bacterium / perikaryon / cytosolic large ribosomal subunit / killing of cells of another organism / cytoplasmic translation / cell differentiation / tRNA binding / postsynapse / mitochondrial inner membrane / postsynaptic density / rRNA binding / structural constituent of ribosome / ribosome / translation / ribonucleoprotein complex / cell division / DNA repair / mRNA binding / apoptotic process / synapse / dendrite / centrosome / negative regulation of apoptotic process / nucleolus / perinuclear region of cytoplasm / endoplasmic reticulum / Golgi apparatus / DNA binding / RNA binding / zinc ion binding / nucleus / membrane / plasma membrane / cytosol / cytoplasm Similarity search - Function | ||||||
| Biological species |  | ||||||
| Method | ELECTRON MICROSCOPY / single particle reconstruction / cryo EM / Resolution: 3.13 Å | ||||||
 Authors Authors | Powers, K.T. / Yadav, S.K.N. / Bufton, J.C. / Schaffitzel, C. | ||||||
| Funding support |  United Kingdom, 1items United Kingdom, 1items
| ||||||
 Citation Citation |  Journal: Nucleic Acids Res / Year: 2021 Journal: Nucleic Acids Res / Year: 2021Title: Blasticidin S inhibits mammalian translation and enhances production of protein encoded by nonsense mRNA. Authors: Kyle T Powers / Flint Stevenson-Jones / Sathish K N Yadav / Beate Amthor / Joshua C Bufton / Ufuk Borucu / Dakang Shen / Jonas P Becker / Daria Lavysh / Matthias W Hentze / Andreas E Kulozik ...Authors: Kyle T Powers / Flint Stevenson-Jones / Sathish K N Yadav / Beate Amthor / Joshua C Bufton / Ufuk Borucu / Dakang Shen / Jonas P Becker / Daria Lavysh / Matthias W Hentze / Andreas E Kulozik / Gabriele Neu-Yilik / Christiane Schaffitzel /   Abstract: Deciphering translation is of paramount importance for the understanding of many diseases, and antibiotics played a pivotal role in this endeavour. Blasticidin S (BlaS) targets translation by binding ...Deciphering translation is of paramount importance for the understanding of many diseases, and antibiotics played a pivotal role in this endeavour. Blasticidin S (BlaS) targets translation by binding to the peptidyl transferase center of the large ribosomal subunit. Using biochemical, structural and cellular approaches, we show here that BlaS inhibits both translation elongation and termination in Mammalia. Bound to mammalian terminating ribosomes, BlaS distorts the 3'CCA tail of the P-site tRNA to a larger extent than previously reported for bacterial ribosomes, thus delaying both, peptide bond formation and peptidyl-tRNA hydrolysis. While BlaS does not inhibit stop codon recognition by the eukaryotic release factor 1 (eRF1), it interferes with eRF1's accommodation into the peptidyl transferase center and subsequent peptide release. In human cells, BlaS inhibits nonsense-mediated mRNA decay and, at subinhibitory concentrations, modulates translation dynamics at premature termination codons leading to enhanced protein production. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  7nwi.cif.gz 7nwi.cif.gz | 7.9 MB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb7nwi.ent.gz pdb7nwi.ent.gz | Display |  PDB format PDB format | |
| PDBx/mmJSON format |  7nwi.json.gz 7nwi.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/nw/7nwi https://data.pdbj.org/pub/pdb/validation_reports/nw/7nwi ftp://data.pdbj.org/pub/pdb/validation_reports/nw/7nwi ftp://data.pdbj.org/pub/pdb/validation_reports/nw/7nwi | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  12633MC  7nwgC  7nwhC M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
+Protein , 32 types, 32 molecules ABFHLOPQSTVXacdefhkorstEEKKOOQQRRTTUUVVff
-60S ribosomal protein ... , 14 types, 14 molecules CDEGIRUWZbgimn
| #3: Protein | Mass: 47727.559 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
|---|---|
| #4: Protein | Mass: 34481.828 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #5: Protein | Mass: 33055.297 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #7: Protein | Mass: 27480.555 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #9: Protein | Mass: 24643.057 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #17: Protein | Mass: 23535.281 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #20: Protein | Mass: 14784.962 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #22: Protein | Mass: 15538.256 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #25: Protein | Mass: 15704.635 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #27: Protein | Mass: 26708.707 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #32: Protein | Mass: 14210.088 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #34: Protein | Mass: 13546.292 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #38: Protein | Mass: 14695.310 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #39: Protein/peptide | Mass: 3213.075 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
-Ribosomal protein ... , 15 types, 15 molecules JMNYjlpFFHHNNSSWWXXddgg
| #10: Protein | Mass: 20670.904 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
|---|---|
| #12: Protein | Mass: 23870.549 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #13: Protein | Mass: 24076.088 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #24: Protein | Mass: 15891.787 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #35: Protein | Mass: 11111.032 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #37: Protein/peptide | Mass: 6295.562 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #41: Protein | Mass: 10168.153 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #50: Protein | Mass: 23044.650 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #52: Protein | Mass: 21716.387 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #58: Protein | Mass: 17128.191 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #63: Protein | Mass: 16170.774 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #67: Protein | Mass: 15778.584 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #68: Protein | Mass: 15626.392 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #74: Protein | Mass: 6364.426 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #77: Protein | Mass: 34669.113 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
-40S ribosomal protein ... , 16 types, 16 molecules AABBCCDDGGIIJJLLMMPPYYZZaabbccee
| #45: Protein | Mass: 32927.988 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
|---|---|
| #46: Protein | Mass: 29942.010 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #47: Protein | Mass: 27956.623 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #48: Protein | Mass: 31146.607 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  References: UniProt: G1TNM3, DNA-(apurinic or apyrimidinic site) lyase |
| #51: Protein | Mass: 30070.432 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #53: Protein | Mass: 24003.012 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #54: Protein | Mass: 22655.590 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #56: Protein | Mass: 18468.826 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #57: Protein | Mass: 13766.122 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #60: Protein | Mass: 17049.182 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #69: Protein | Mass: 17007.125 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #70: Protein | Mass: 13581.994 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #71: Protein | Mass: 13147.561 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #72: Protein | Mass: 9480.186 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #73: Protein | Mass: 7776.891 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #75: Protein | Mass: 14498.884 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
-Protein/peptide , 1 types, 1 molecules 1
| #78: Protein/peptide | Mass: 1788.032 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
|---|
-RNA chain , 6 types, 6 molecules 25789K
| #79: RNA chain | Mass: 24436.551 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
|---|---|
| #80: RNA chain | Mass: 1226739.500 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #81: RNA chain | Mass: 38691.914 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #82: RNA chain | Mass: 50143.648 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #83: RNA chain | Mass: 573221.562 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #84: RNA chain | Mass: 3225.980 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
-Non-polymers , 3 types, 216 molecules 

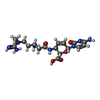


| #85: Chemical | ChemComp-MG / #86: Chemical | ChemComp-ZN / #87: Chemical | ChemComp-BLS / | |
|---|
-Details
| Has ligand of interest | Y |
|---|---|
| Has protein modification | Y |
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method: single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: Mammalian pre-termination 80S ribosome with Empty-A site bound by Blasticidin S Type: RIBOSOME Details: Rabbit reticulocyte lysate derived (in vitro transcription-FLAG affinity purified) ribosomal complex. Entity ID: #1-#2, #4-#8, #10-#14, #16-#20, #22-#31, #33-#34, #36-#37, #39, #42-#79 Source: NATURAL |
|---|---|
| Molecular weight | Value: 3.8 MDa / Experimental value: NO |
| Source (natural) | Organism:  |
| Buffer solution | pH: 7.4 Details: 50 mM HEPES pH 7.4 100 mM KOAc 5 mM Mg(Oac)2 1mM DTT |
| Specimen | Conc.: 0.63 mg/ml / Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: YES / Details: 165nM (OD260nm~10.5) |
| Specimen support | Grid material: COPPER / Grid mesh size: 300 divisions/in. / Grid type: Quantifoil R2/2 |
| Vitrification | Instrument: LEICA EM GP / Cryogen name: ETHANE / Humidity: 70 % / Chamber temperature: 288.15 K Details: 30s sample incubation on grid followed by 1.1s blotting time. |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Talos Arctica / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TALOS ARCTICA Details: Collected in super-resolution mode (0.675A pixel size) |
| Electron gun | Electron source:  FIELD EMISSION GUN / Accelerating voltage: 200 kV / Illumination mode: OTHER FIELD EMISSION GUN / Accelerating voltage: 200 kV / Illumination mode: OTHER |
| Electron lens | Mode: BRIGHT FIELD / Calibrated magnification: 79000 X / Calibrated defocus min: 400 nm / Calibrated defocus max: 2000 nm / Cs: 2.7 mm / C2 aperture diameter: 100 µm / Alignment procedure: COMA FREE |
| Specimen holder | Cryogen: NITROGEN / Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER |
| Image recording | Average exposure time: 8 sec. / Electron dose: 41.92 e/Å2 / Detector mode: SUPER-RESOLUTION / Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Num. of grids imaged: 1 / Num. of real images: 3500 |
| Image scans | Movie frames/image: 40 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EM software |
| ||||||||||||||||||||||||||||||||
| CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION | ||||||||||||||||||||||||||||||||
| Particle selection | Num. of particles selected: 730463 | ||||||||||||||||||||||||||||||||
| Symmetry | Point symmetry: C1 (asymmetric) | ||||||||||||||||||||||||||||||||
| 3D reconstruction | Resolution: 3.13 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 103842 / Num. of class averages: 1 / Symmetry type: POINT | ||||||||||||||||||||||||||||||||
| Atomic model building | Protocol: RIGID BODY FIT | ||||||||||||||||||||||||||||||||
| Atomic model building | PDB-ID: 3JAH Accession code: 3JAH / Source name: PDB / Type: experimental model | ||||||||||||||||||||||||||||||||
| Refinement | Stereochemistry target values: GeoStd + Monomer Library | ||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller














 PDBj
PDBj



































