+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 7nmp | ||||||
|---|---|---|---|---|---|---|---|
| Title | E. coli NfsA with hydroquinone | ||||||
 Components Components | Oxygen-insensitive NADPH nitroreductase | ||||||
 Keywords Keywords | OXIDOREDUCTASE / complex with quinone product / flavoprotein / nitroreductase | ||||||
| Function / homology |  Function and homology information Function and homology informationNAD(P)H dehydrogenase (quinone) activity / Oxidoreductases / FMN binding / protein homodimerization activity / cytosol Similarity search - Function | ||||||
| Biological species |  | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  MOLECULAR REPLACEMENT / Resolution: 1.25 Å MOLECULAR REPLACEMENT / Resolution: 1.25 Å | ||||||
 Authors Authors | Day, M.D. / Jarrom, D. / Parr, R.J. / Hyde, E.I. / White, S.A. | ||||||
 Citation Citation |  Journal: Biochem.J. / Year: 2021 Journal: Biochem.J. / Year: 2021Title: The structures of E. coli NfsA bound to the antibiotic nitrofurantoin; to 1,4-benzoquinone and to FMN. Authors: Day, M.A. / Jarrom, D. / Christofferson, A.J. / Graziano, A.E. / Anderson, J.L.R. / Searle, P.F. / Hyde, E.I. / White, S.A. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  7nmp.cif.gz 7nmp.cif.gz | 132.7 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb7nmp.ent.gz pdb7nmp.ent.gz | 102.2 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  7nmp.json.gz 7nmp.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  7nmp_validation.pdf.gz 7nmp_validation.pdf.gz | 423.9 KB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  7nmp_full_validation.pdf.gz 7nmp_full_validation.pdf.gz | 424.1 KB | Display | |
| Data in XML |  7nmp_validation.xml.gz 7nmp_validation.xml.gz | 14.3 KB | Display | |
| Data in CIF |  7nmp_validation.cif.gz 7nmp_validation.cif.gz | 22 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/nm/7nmp https://data.pdbj.org/pub/pdb/validation_reports/nm/7nmp ftp://data.pdbj.org/pub/pdb/validation_reports/nm/7nmp ftp://data.pdbj.org/pub/pdb/validation_reports/nm/7nmp | HTTPS FTP |
-Related structure data
| Related structure data |  7nb9C  7niyC  7nnxC  1f5vS C: citing same article ( S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| Unit cell |
| ||||||||
| Components on special symmetry positions |
|
- Components
Components
-Protein , 1 types, 1 molecules A
| #1: Protein | Mass: 26832.664 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Strain: K12 / Gene: nfsA, mda18, mdaA, ybjB, b0851, JW0835 / Plasmid: pPS1341A1 / Details (production host): pET24 derivative / Production host:  |
|---|
-Non-polymers , 6 types, 294 molecules 



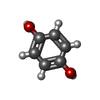






| #2: Chemical | ChemComp-FMN / | ||||||
|---|---|---|---|---|---|---|---|
| #3: Chemical | ChemComp-DMS / | ||||||
| #4: Chemical | | #5: Chemical | ChemComp-CL / | #6: Chemical | ChemComp-HQE / | #7: Water | ChemComp-HOH / | |
-Details
| Has ligand of interest | Y |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.07 Å3/Da / Density % sol: 40.63 % |
|---|---|
| Crystal grow | Temperature: 291 K / Method: vapor diffusion, sitting drop / pH: 7 Details: 28% PEG 3,000; 100 mM imidazole pH 7.0; 20% DMSO,25 mM hydroxyquinone |
-Data collection
| Diffraction | Mean temperature: 100 K / Ambient temp details: 291 / Serial crystal experiment: N |
|---|---|
| Diffraction source | Source:  ROTATING ANODE / Type: RIGAKU / Wavelength: 1.54 Å ROTATING ANODE / Type: RIGAKU / Wavelength: 1.54 Å |
| Detector | Type: RIGAKU SATURN 944 / Detector: CCD / Date: May 11, 2011 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1.54 Å / Relative weight: 1 |
| Reflection | Resolution: 1.25→46.46 Å / Num. obs: 59285 / % possible obs: 97.6 % / Redundancy: 6.39 % / Rsym value: 0.05 / Net I/σ(I): 19.2 |
| Reflection shell | Resolution: 1.25→1.35 Å / Mean I/σ(I) obs: 2.4 / Num. unique obs: 25991 / Rsym value: 0.54 / % possible all: 84.9 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 1F5V Resolution: 1.25→46.03 Å / Cor.coef. Fo:Fc: 0.98 / Cor.coef. Fo:Fc free: 0.971 / SU B: 1.533 / SU ML: 0.029 / Cross valid method: THROUGHOUT / ESU R: 0.042 / ESU R Free: 0.041 / Stereochemistry target values: MAXIMUM LIKELIHOOD Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS U VALUES : REFINED INDIVIDUALLY SEQRES 1 A 240 MET THR PRO THR ILE GLU LEU ILE CYS GLY HIS ARG SER
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.2 Å / Solvent model: MASK | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso max: 61.66 Å2 / Biso mean: 16.149 Å2 / Biso min: 6.54 Å2
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: final / Resolution: 1.25→46.03 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 1.25→1.283 Å / Rfactor Rfree error: 0 / Total num. of bins used: 20
|
 Movie
Movie Controller
Controller













 PDBj
PDBj




