+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 7mxn | ||||||
|---|---|---|---|---|---|---|---|
| Title | PRMT5(M420T mutant):MEP50 complexed with inhibitor PF-06939999 | ||||||
 Components Components |
| ||||||
 Keywords Keywords | transferase/Transcription / arginine / methyl / transferase / transferase-Transcription complex | ||||||
| Function / homology |  Function and homology information Function and homology informationpositive regulation of adenylate cyclase-inhibiting dopamine receptor signaling pathway / peptidyl-arginine N-methylation / oocyte axis specification / type II protein arginine methyltransferase / protein-arginine omega-N symmetric methyltransferase activity / peptidyl-arginine methylation / Golgi ribbon formation / negative regulation of epithelial cell proliferation involved in prostate gland development / secretory columnal luminar epithelial cell differentiation involved in prostate glandular acinus development / : ...positive regulation of adenylate cyclase-inhibiting dopamine receptor signaling pathway / peptidyl-arginine N-methylation / oocyte axis specification / type II protein arginine methyltransferase / protein-arginine omega-N symmetric methyltransferase activity / peptidyl-arginine methylation / Golgi ribbon formation / negative regulation of epithelial cell proliferation involved in prostate gland development / secretory columnal luminar epithelial cell differentiation involved in prostate glandular acinus development / : / histone H4R3 methyltransferase activity / epithelial cell proliferation involved in prostate gland development / protein-arginine N-methyltransferase activity / methylosome / positive regulation of mRNA splicing, via spliceosome / methyl-CpG binding / endothelial cell activation / histone H3 methyltransferase activity / regulation of mitotic nuclear division / histone methyltransferase activity / negative regulation of gene expression via chromosomal CpG island methylation / Cul4B-RING E3 ubiquitin ligase complex / E-box binding / histone methyltransferase complex / positive regulation of oligodendrocyte differentiation / negative regulation of cell differentiation / spliceosomal snRNP assembly / ribonucleoprotein complex binding / regulation of ERK1 and ERK2 cascade / ubiquitin-like ligase-substrate adaptor activity / liver regeneration / regulation of signal transduction by p53 class mediator / methyltransferase activity / circadian regulation of gene expression / DNA-templated transcription termination / Regulation of TP53 Activity through Methylation / RMTs methylate histone arginines / protein polyubiquitination / p53 binding / transcription corepressor activity / snRNP Assembly / ubiquitin-dependent protein catabolic process / transcription coactivator activity / chromatin remodeling / protein heterodimerization activity / positive regulation of cell population proliferation / regulation of transcription by RNA polymerase II / regulation of DNA-templated transcription / chromatin / Golgi apparatus / nucleoplasm / identical protein binding / nucleus / cytoplasm / cytosol Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  FOURIER SYNTHESIS / Resolution: 2.55 Å FOURIER SYNTHESIS / Resolution: 2.55 Å | ||||||
 Authors Authors | McTigue, M. / Deng, Y.L. / Liu, W. / Brooun, A. | ||||||
 Citation Citation |  Journal: Mol.Cancer Ther. / Year: 2022 Journal: Mol.Cancer Ther. / Year: 2022Title: SAM-Competitive PRMT5 Inhibitor PF-06939999 Demonstrates Antitumor Activity in Splicing Dysregulated NSCLC with Decreased Liability of Drug Resistance. Authors: Jensen-Pergakes, K. / Tatlock, J. / Maegley, K.A. / McAlpine, I.J. / McTigue, M. / Xie, T. / Dillon, C.P. / Wang, Y. / Yamazaki, S. / Spiegel, N. / Shi, M. / Nemeth, A. / Miller, N. / ...Authors: Jensen-Pergakes, K. / Tatlock, J. / Maegley, K.A. / McAlpine, I.J. / McTigue, M. / Xie, T. / Dillon, C.P. / Wang, Y. / Yamazaki, S. / Spiegel, N. / Shi, M. / Nemeth, A. / Miller, N. / Hendrickson, E. / Lam, H. / Sherrill, J. / Chung, C.Y. / McMillan, E.A. / Bryant, S.K. / Palde, P. / Braganza, J. / Brooun, A. / Deng, Y.L. / Goshtasbi, V. / Kephart, S.E. / Kumpf, R.A. / Liu, W. / Patman, R.L. / Rui, E. / Scales, S. / Tran-Dube, M. / Wang, F. / Wythes, M. / Paul, T.A. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  7mxn.cif.gz 7mxn.cif.gz | 202.6 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb7mxn.ent.gz pdb7mxn.ent.gz | 157.3 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  7mxn.json.gz 7mxn.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/mx/7mxn https://data.pdbj.org/pub/pdb/validation_reports/mx/7mxn ftp://data.pdbj.org/pub/pdb/validation_reports/mx/7mxn ftp://data.pdbj.org/pub/pdb/validation_reports/mx/7mxn | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  7mx7SC  7mxaC  7mxcC  7mxgC S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 72736.570 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: PRMT5, HRMT1L5, IBP72, JBP1, SKB1 / Production host: Homo sapiens (human) / Gene: PRMT5, HRMT1L5, IBP72, JBP1, SKB1 / Production host:  References: UniProt: O14744, type II protein arginine methyltransferase |
|---|---|
| #2: Protein | Mass: 39520.082 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: WDR77, MEP50, WD45, HKMT1069, Nbla10071 / Production host: Homo sapiens (human) / Gene: WDR77, MEP50, WD45, HKMT1069, Nbla10071 / Production host:  |
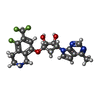
| #3: Chemical | ChemComp-ZR1 / ( |
| #4: Water | ChemComp-HOH / |
| Has ligand of interest | Y |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.77 Å3/Da / Density % sol: 55.55 % |
|---|---|
| Crystal grow | Temperature: 286 K / Method: vapor diffusion, hanging drop Details: Crystallization of full-length human PRMT5/MEP50 complexed with cofactor site inhibitors was performed at 13 degrees Celsius by hanging-drop vapor-diffusion methods. 2.5 ul of a solution of ...Details: Crystallization of full-length human PRMT5/MEP50 complexed with cofactor site inhibitors was performed at 13 degrees Celsius by hanging-drop vapor-diffusion methods. 2.5 ul of a solution of 5:1 molar ratio of inhibitor compound to PRMT5/MEP50 complex (13 mg/mL) was mixed with 2.5 ul of reservoir solution containing 13-15% (w/v) PEG3350, 0.1M MES, pH 6.5-7.5, 0.25M NaCl, and 20% (v/v) ethylene glycol. Microseeding from initial crystals produced crystals suitable for data collection. |
-Data collection
| Diffraction | Mean temperature: 95 K / Serial crystal experiment: N |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  APS APS  / Beamline: 17-ID / Wavelength: 1 Å / Beamline: 17-ID / Wavelength: 1 Å |
| Detector | Type: DECTRIS PILATUS 6M / Detector: PIXEL / Date: Oct 4, 2017 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1 Å / Relative weight: 1 |
| Reflection | Resolution: 2.55→109.19 Å / Num. obs: 41147 / % possible obs: 99.9 % / Redundancy: 6.6 % / Rmerge(I) obs: 0.073 / Rpim(I) all: 0.043 / Net I/σ(I): 13.3 |
| Reflection shell | Resolution: 2.55→2.68 Å / Redundancy: 6.9 % / Rmerge(I) obs: 0.475 / Mean I/σ(I) obs: 2.6 / Num. unique obs: 5958 / Rpim(I) all: 0.194 / Rrim(I) all: 0.514 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  FOURIER SYNTHESIS FOURIER SYNTHESISStarting model: 7MX7 Resolution: 2.55→109.19 Å / Cor.coef. Fo:Fc: 0.833 / Cor.coef. Fo:Fc free: 0.807 / SU R Cruickshank DPI: 0.502 / Cross valid method: THROUGHOUT / SU R Blow DPI: 0.505 / SU Rfree Blow DPI: 0.298 / SU Rfree Cruickshank DPI: 0.302
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 78.34 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine analyze | Luzzati coordinate error obs: 0.46 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.55→109.19 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 2.55→2.56 Å
|
 Movie
Movie Controller
Controller























 PDBj
PDBj