[English] 日本語
 Yorodumi
Yorodumi- PDB-7li8: apo serotonin transporter reconstituted in lipid nanodisc in pres... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 7li8 | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | apo serotonin transporter reconstituted in lipid nanodisc in presence of NaCl in inward open conformation | |||||||||||||||||||||||||||
 Components Components |
| |||||||||||||||||||||||||||
 Keywords Keywords | MEMBRANE PROTEIN / transport / serotonin transporter / Fab / inward | |||||||||||||||||||||||||||
| Function / homology |  Function and homology information Function and homology informationnegative regulation of cerebellar granule cell precursor proliferation / regulation of thalamus size / Serotonin clearance from the synaptic cleft / positive regulation of serotonin secretion / serotonergic synapse / negative regulation of synaptic transmission, dopaminergic / cocaine binding / serotonin:sodium:chloride symporter activity / cellular response to cGMP / enteric nervous system development ...negative regulation of cerebellar granule cell precursor proliferation / regulation of thalamus size / Serotonin clearance from the synaptic cleft / positive regulation of serotonin secretion / serotonergic synapse / negative regulation of synaptic transmission, dopaminergic / cocaine binding / serotonin:sodium:chloride symporter activity / cellular response to cGMP / enteric nervous system development / sodium ion binding / negative regulation of organ growth / neurotransmitter transmembrane transporter activity / serotonin uptake / vasoconstriction / monoamine transmembrane transporter activity / monoamine transport / serotonin binding / brain morphogenesis / antiporter activity / syntaxin-1 binding / neurotransmitter transport / male mating behavior / negative regulation of neuron differentiation / nitric-oxide synthase binding / amino acid transport / conditioned place preference / membrane depolarization / monoatomic cation channel activity / behavioral response to cocaine / positive regulation of cell cycle / cellular response to retinoic acid / response to nutrient / sodium ion transmembrane transport / endomembrane system / circadian rhythm / response to toxic substance / platelet aggregation / memory / integrin binding / actin filament binding / response to estradiol / presynaptic membrane / postsynaptic membrane / response to hypoxia / neuron projection / endosome membrane / membrane raft / response to xenobiotic stimulus / focal adhesion / synapse / positive regulation of gene expression / identical protein binding / plasma membrane Similarity search - Function | |||||||||||||||||||||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||||||||||||||||||||
| Method | ELECTRON MICROSCOPY / single particle reconstruction / cryo EM / Resolution: 3.9 Å | |||||||||||||||||||||||||||
 Authors Authors | Yang, D. / Gouaux, E. | |||||||||||||||||||||||||||
| Funding support |  United States, 2items United States, 2items
| |||||||||||||||||||||||||||
 Citation Citation |  Journal: Sci Adv / Year: 2021 Journal: Sci Adv / Year: 2021Title: Illumination of serotonin transporter mechanism and role of the allosteric site. Authors: Dongxue Yang / Eric Gouaux /  Abstract: The serotonin transporter (SERT) terminates serotonin signaling by using sodium and chloride gradients to drive reuptake of serotonin into presynaptic neurons and is the target of widely used ...The serotonin transporter (SERT) terminates serotonin signaling by using sodium and chloride gradients to drive reuptake of serotonin into presynaptic neurons and is the target of widely used medications to treat neuropsychiatric disorders. Despite decades of study, the molecular mechanism of serotonin transport, the coupling to ion gradients, and the role of the allosteric site have remained elusive. Here, we present cryo–electron microscopy structures of SERT in serotonin-bound and serotonin-free states, in the presence of sodium or potassium, resolving all fundamental states of the transport cycle. From the SERT-serotonin complex, we localize the substrate-bound allosteric site, formed by an aromatic pocket positioned in the scaffold domain in the extracellular vestibule, connected to the central site via a short tunnel. Together with elucidation of multiple apo state conformations, we provide previously unseen structural understanding of allosteric modulation, demonstrating how SERT binds serotonin from synaptic volumes and promotes unbinding into the presynaptic neurons. | |||||||||||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  7li8.cif.gz 7li8.cif.gz | 152.6 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb7li8.ent.gz pdb7li8.ent.gz | 115.2 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  7li8.json.gz 7li8.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  7li8_validation.pdf.gz 7li8_validation.pdf.gz | 912.7 KB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  7li8_full_validation.pdf.gz 7li8_full_validation.pdf.gz | 919.6 KB | Display | |
| Data in XML |  7li8_validation.xml.gz 7li8_validation.xml.gz | 28.6 KB | Display | |
| Data in CIF |  7li8_validation.cif.gz 7li8_validation.cif.gz | 41.1 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/li/7li8 https://data.pdbj.org/pub/pdb/validation_reports/li/7li8 ftp://data.pdbj.org/pub/pdb/validation_reports/li/7li8 ftp://data.pdbj.org/pub/pdb/validation_reports/li/7li8 | HTTPS FTP |
-Related structure data
| Related structure data |  23363MC  7li6C  7li7C  7li9C  7liaC  7mgwC M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
-Antibody , 2 types, 2 molecules BC
| #2: Antibody | Mass: 12980.533 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
|---|---|
| #3: Antibody | Mass: 11776.065 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
-Protein / Sugars , 2 types, 2 molecules A
| #1: Protein | Mass: 60875.957 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: SLC6A4, HTT, SERT / Production host: Homo sapiens (human) / Gene: SLC6A4, HTT, SERT / Production host:  Homo sapiens (human) / References: UniProt: P31645 Homo sapiens (human) / References: UniProt: P31645 |
|---|---|
| #4: Polysaccharide | 2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose Source method: isolated from a genetically manipulated source |
-Non-polymers , 5 types, 12 molecules 

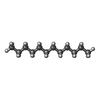
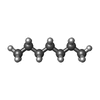
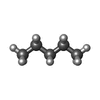



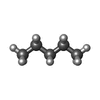
| #5: Chemical | ChemComp-R16 / | ||||
|---|---|---|---|---|---|
| #6: Chemical | ChemComp-D10 / | ||||
| #7: Chemical | | #8: Chemical | ChemComp-HP6 / #9: Chemical | ChemComp-LNK / | |
-Details
| Has ligand of interest | N |
|---|---|
| Has protein modification | Y |
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method: single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: apo human serotonin transporter in complex with 15B8 Fab in NaCl Type: COMPLEX / Entity ID: #1-#3 / Source: MULTIPLE SOURCES | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Source (natural) |
| ||||||||||||
| Source (recombinant) | Organism:  Homo sapiens (human) Homo sapiens (human) | ||||||||||||
| Buffer solution | pH: 8 | ||||||||||||
| Specimen | Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: YES | ||||||||||||
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TITAN KRIOS |
| Electron gun | Electron source:  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD |
| Image recording | Electron dose: 50 e/Å2 / Film or detector model: GATAN K3 (6k x 4k) |
- Processing
Processing
| Software | Name: PHENIX / Version: 1.17_3644: / Classification: refinement | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EM software | Name: PHENIX / Category: model refinement | ||||||||||||||||||||||||
| CTF correction | Type: PHASE FLIPPING ONLY | ||||||||||||||||||||||||
| 3D reconstruction | Resolution: 3.9 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 343085 / Symmetry type: POINT | ||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller

















 PDBj
PDBj









