[English] 日本語
 Yorodumi
Yorodumi- EMDB-23363: apo serotonin transporter reconstituted in lipid nanodisc in pres... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-23363 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | apo serotonin transporter reconstituted in lipid nanodisc in presence of NaCl in inward open conformation | |||||||||
 Map data Map data | sharpened map | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | transport / serotonin transporter / Fab / inward / MEMBRANE PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationnegative regulation of cerebellar granule cell precursor proliferation / regulation of thalamus size / Serotonin clearance from the synaptic cleft / positive regulation of serotonin secretion / serotonergic synapse / negative regulation of synaptic transmission, dopaminergic / cocaine binding / serotonin:sodium:chloride symporter activity / cellular response to cGMP / enteric nervous system development ...negative regulation of cerebellar granule cell precursor proliferation / regulation of thalamus size / Serotonin clearance from the synaptic cleft / positive regulation of serotonin secretion / serotonergic synapse / negative regulation of synaptic transmission, dopaminergic / cocaine binding / serotonin:sodium:chloride symporter activity / cellular response to cGMP / enteric nervous system development / negative regulation of organ growth / sodium ion binding / neurotransmitter transmembrane transporter activity / serotonin uptake / vasoconstriction / monoamine transmembrane transporter activity / monoamine transport / serotonin binding / brain morphogenesis / syntaxin-1 binding / antiporter activity / negative regulation of neuron differentiation / male mating behavior / neurotransmitter transport / nitric-oxide synthase binding / amino acid transport / conditioned place preference / membrane depolarization / monoatomic cation channel activity / behavioral response to cocaine / cellular response to retinoic acid / positive regulation of cell cycle / endomembrane system / response to nutrient / sodium ion transmembrane transport / circadian rhythm / platelet aggregation / response to toxic substance / integrin binding / memory / actin filament binding / response to estradiol / presynaptic membrane / postsynaptic membrane / response to hypoxia / neuron projection / endosome membrane / membrane raft / response to xenobiotic stimulus / focal adhesion / synapse / positive regulation of gene expression / identical protein binding / plasma membrane Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) / Homo sapiens (human) /  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.9 Å | |||||||||
 Authors Authors | Yang D / Gouaux E | |||||||||
| Funding support |  United States, 2 items United States, 2 items
| |||||||||
 Citation Citation |  Journal: Sci Adv / Year: 2021 Journal: Sci Adv / Year: 2021Title: Illumination of serotonin transporter mechanism and role of the allosteric site. Authors: Dongxue Yang / Eric Gouaux /  Abstract: The serotonin transporter (SERT) terminates serotonin signaling by using sodium and chloride gradients to drive reuptake of serotonin into presynaptic neurons and is the target of widely used ...The serotonin transporter (SERT) terminates serotonin signaling by using sodium and chloride gradients to drive reuptake of serotonin into presynaptic neurons and is the target of widely used medications to treat neuropsychiatric disorders. Despite decades of study, the molecular mechanism of serotonin transport, the coupling to ion gradients, and the role of the allosteric site have remained elusive. Here, we present cryo–electron microscopy structures of SERT in serotonin-bound and serotonin-free states, in the presence of sodium or potassium, resolving all fundamental states of the transport cycle. From the SERT-serotonin complex, we localize the substrate-bound allosteric site, formed by an aromatic pocket positioned in the scaffold domain in the extracellular vestibule, connected to the central site via a short tunnel. Together with elucidation of multiple apo state conformations, we provide previously unseen structural understanding of allosteric modulation, demonstrating how SERT binds serotonin from synaptic volumes and promotes unbinding into the presynaptic neurons. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_23363.map.gz emd_23363.map.gz | 229.6 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-23363-v30.xml emd-23363-v30.xml emd-23363.xml emd-23363.xml | 17 KB 17 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_23363.png emd_23363.png | 101.1 KB | ||
| Filedesc metadata |  emd-23363.cif.gz emd-23363.cif.gz | 6.5 KB | ||
| Others |  emd_23363_additional_1.map.gz emd_23363_additional_1.map.gz | 119.7 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-23363 http://ftp.pdbj.org/pub/emdb/structures/EMD-23363 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-23363 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-23363 | HTTPS FTP |
-Validation report
| Summary document |  emd_23363_validation.pdf.gz emd_23363_validation.pdf.gz | 466.8 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_23363_full_validation.pdf.gz emd_23363_full_validation.pdf.gz | 466.4 KB | Display | |
| Data in XML |  emd_23363_validation.xml.gz emd_23363_validation.xml.gz | 7.1 KB | Display | |
| Data in CIF |  emd_23363_validation.cif.gz emd_23363_validation.cif.gz | 8.2 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-23363 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-23363 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-23363 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-23363 | HTTPS FTP |
-Related structure data
| Related structure data |  7li8MC  7li6C  7li7C  7li9C  7liaC  7mgwC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_23363.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_23363.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | sharpened map | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.648 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Additional map: unsharpened map
| File | emd_23363_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | unsharpened map | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : apo human serotonin transporter in complex with 15B8 Fab in NaCl
| Entire | Name: apo human serotonin transporter in complex with 15B8 Fab in NaCl |
|---|---|
| Components |
|
-Supramolecule #1: apo human serotonin transporter in complex with 15B8 Fab in NaCl
| Supramolecule | Name: apo human serotonin transporter in complex with 15B8 Fab in NaCl type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#3 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Sodium-dependent serotonin transporter
| Macromolecule | Name: Sodium-dependent serotonin transporter / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 60.875957 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: RETWGKKVDF LLSVIGYAVD LGNVWRFPYI CYQNGGGAFL LPYTIMAIFG GIPLFYMELA LGQYHRNGCI SIWRKICPIF KGIGYAICI IAFYIASYYN TIMAWALYYL ISSFTDQLPW TSCKNSWNTG NCTNYFSEDN ITWTLHSTSP AEEFYTRHVL Q IHRSKGLQ ...String: RETWGKKVDF LLSVIGYAVD LGNVWRFPYI CYQNGGGAFL LPYTIMAIFG GIPLFYMELA LGQYHRNGCI SIWRKICPIF KGIGYAICI IAFYIASYYN TIMAWALYYL ISSFTDQLPW TSCKNSWNTG NCTNYFSEDN ITWTLHSTSP AEEFYTRHVL Q IHRSKGLQ DLGGISWQLA LCIMLIFTVI YFSIWKGVKT SGKVVWVTAT FPYIILSVLL VRGATLPGAW RGVLFYLKPN WQ KLLETGV WIDAAAQIFF SLGPGFGVLL AFASYNKFNN NCYQDALVTS VVNCMTSFVS GFVIFTVLGY MAEMRNEDVS EVA KDAGPS LLFITYAEAI ANMPASTFFA IIFFLMLITL GLDSTFAGLE GVITAVLDEF PHVWAKRRER FVLAVVITCF FGSL VTLTF GGAYVVKLLE EYATGPAVLT VALIEAVAVS WFYGITQFCR DVKEMLGFSP GWFWRICWVA ISPLFLLFII CSFLM SPPQ LRLFQYNYPY WSIILGYCIG TSSFICIPTY IAYRLIITPG TFKERIIKSI TPETP UniProtKB: Sodium-dependent serotonin transporter |
-Macromolecule #2: variable domain of 15B8 antibody Fab heavy chain
| Macromolecule | Name: variable domain of 15B8 antibody Fab heavy chain / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 12.980533 KDa |
| Sequence | String: QVQLQQSGPE LVKLGASVRI SCKASGYRFS YSWMNWVKQR PGKGLEWIGR IYPGDGDTKY SGKFKGKATL TADKSSSTVY MQLSSLTSE DSAVYFCARS AYGSEGFAMD YWGQGTSVT |
-Macromolecule #3: variable domain of 15B8 antibody Fab light chain
| Macromolecule | Name: variable domain of 15B8 antibody Fab light chain / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 11.776065 KDa |
| Sequence | String: DIVLTQSPAS LAVSLGQRAT ISCRASESVD NYGISFLNWF QQKPGQPPKL LIYAASNQGS GVPARFSGSG SGTYFSLNIH PMEEDDTAV YFCQQTKGVS WTFGGGTKVE I |
-Macromolecule #5: HEXADECANE
| Macromolecule | Name: HEXADECANE / type: ligand / ID: 5 / Number of copies: 1 / Formula: R16 |
|---|---|
| Molecular weight | Theoretical: 226.441 Da |
| Chemical component information |  ChemComp-R16: |
-Macromolecule #6: DECANE
| Macromolecule | Name: DECANE / type: ligand / ID: 6 / Number of copies: 1 / Formula: D10 |
|---|---|
| Molecular weight | Theoretical: 142.282 Da |
| Chemical component information |  ChemComp-D10: |
-Macromolecule #7: DODECANE
| Macromolecule | Name: DODECANE / type: ligand / ID: 7 / Number of copies: 3 / Formula: D12 |
|---|---|
| Molecular weight | Theoretical: 170.335 Da |
| Chemical component information |  ChemComp-D12: |
-Macromolecule #8: HEPTANE
| Macromolecule | Name: HEPTANE / type: ligand / ID: 8 / Number of copies: 6 / Formula: HP6 |
|---|---|
| Molecular weight | Theoretical: 100.202 Da |
| Chemical component information |  ChemComp-HP6: |
-Macromolecule #9: PENTANE
| Macromolecule | Name: PENTANE / type: ligand / ID: 9 / Number of copies: 1 / Formula: LNK |
|---|---|
| Molecular weight | Theoretical: 72.149 Da |
| Chemical component information | 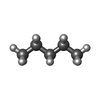 ChemComp-LNK: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 8 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller



















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)