+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 7kq0 | ||||||
|---|---|---|---|---|---|---|---|
| Title | PCNA bound to peptide mimetic | ||||||
 Components Components |
| ||||||
 Keywords Keywords | REPLICATION / PCNA / DNA replication / peptide mimetic | ||||||
| Function / homology |  Function and homology information Function and homology informationpositive regulation of deoxyribonuclease activity / dinucleotide insertion or deletion binding / PCNA-p21 complex / mitotic telomere maintenance via semi-conservative replication / purine-specific mismatch base pair DNA N-glycosylase activity / nuclear lamina / positive regulation of DNA-directed DNA polymerase activity / Polymerase switching / Processive synthesis on the lagging strand / MutLalpha complex binding ...positive regulation of deoxyribonuclease activity / dinucleotide insertion or deletion binding / PCNA-p21 complex / mitotic telomere maintenance via semi-conservative replication / purine-specific mismatch base pair DNA N-glycosylase activity / nuclear lamina / positive regulation of DNA-directed DNA polymerase activity / Polymerase switching / Processive synthesis on the lagging strand / MutLalpha complex binding / PCNA complex / Removal of the Flap Intermediate / Telomere C-strand (Lagging Strand) Synthesis / Mismatch repair (MMR) directed by MSH2:MSH3 (MutSbeta) / Mismatch repair (MMR) directed by MSH2:MSH6 (MutSalpha) / Transcription of E2F targets under negative control by DREAM complex / Polymerase switching on the C-strand of the telomere / replisome / response to L-glutamate / Processive synthesis on the C-strand of the telomere / Removal of the Flap Intermediate from the C-strand / response to dexamethasone / histone acetyltransferase binding / DNA polymerase processivity factor activity / G1/S-Specific Transcription / leading strand elongation / nuclear replication fork / replication fork processing / SUMOylation of DNA replication proteins / PCNA-Dependent Long Patch Base Excision Repair / response to cadmium ion / translesion synthesis / estrous cycle / mismatch repair / cyclin-dependent protein kinase holoenzyme complex / base-excision repair, gap-filling / DNA polymerase binding / epithelial cell differentiation / liver regeneration / TP53 Regulates Transcription of Genes Involved in G2 Cell Cycle Arrest / positive regulation of DNA repair / positive regulation of DNA replication / Translesion synthesis by REV1 / nuclear estrogen receptor binding / replication fork / Translesion synthesis by POLK / Translesion synthesis by POLI / Gap-filling DNA repair synthesis and ligation in GG-NER / male germ cell nucleus / Termination of translesion DNA synthesis / Translesion Synthesis by POLH / Recognition of DNA damage by PCNA-containing replication complex / receptor tyrosine kinase binding / HDR through Homologous Recombination (HRR) / cellular response to xenobiotic stimulus / Dual Incision in GG-NER / cellular response to hydrogen peroxide / Dual incision in TC-NER / Gap-filling DNA repair synthesis and ligation in TC-NER / cellular response to UV / response to estradiol / E3 ubiquitin ligases ubiquitinate target proteins / heart development / chromatin organization / damaged DNA binding / chromosome, telomeric region / nuclear body / chromatin binding / centrosome / chromatin / protein-containing complex binding / enzyme binding / negative regulation of transcription by RNA polymerase II / extracellular exosome / nucleoplasm / identical protein binding / nucleus Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human)synthetic construct (others) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.4 Å MOLECULAR REPLACEMENT / Resolution: 2.4 Å | ||||||
 Authors Authors | Vandborg, B.A. / Bruning, J.B. | ||||||
 Citation Citation |  Journal: J.Biol.Chem. / Year: 2021 Journal: J.Biol.Chem. / Year: 2021Title: Unlocking the PIP-box: A peptide library reveals interactions that drive high-affinity binding to human PCNA. Authors: Horsfall, A.J. / Vandborg, B.A. / Kowalczyk, W. / Chav, T. / Scanlon, D.B. / Abell, A.D. / Bruning, J.B. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  7kq0.cif.gz 7kq0.cif.gz | 163.9 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb7kq0.ent.gz pdb7kq0.ent.gz | 127.8 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  7kq0.json.gz 7kq0.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/kq/7kq0 https://data.pdbj.org/pub/pdb/validation_reports/kq/7kq0 ftp://data.pdbj.org/pub/pdb/validation_reports/kq/7kq0 ftp://data.pdbj.org/pub/pdb/validation_reports/kq/7kq0 | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  7kq1C  4rjfS S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2 | 
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3 | 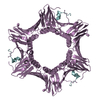
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Unit cell |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Noncrystallographic symmetry (NCS) | NCS domain:
NCS domain segments:
|
 Movie
Movie Controller
Controller











 PDBj
PDBj














