+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 7fef | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal structure of AtMBD6 with DNA | ||||||
 Components Components |
| ||||||
 Keywords Keywords | DNA BINDING PROTEIN/DNA / MBD / DNA-protein complex structure / DNA BINDING PROTEIN / DNA BINDING PROTEIN-DNA complex | ||||||
| Function / homology |  Function and homology information Function and homology informationnucleolus organizer region / perinucleolar chromocenter / methyl-CpG binding / regulatory ncRNA-mediated gene silencing / plastid / heterochromatin / enzyme binding / nucleus Similarity search - Function | ||||||
| Biological species |  synthetic construct (others) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.39 Å MOLECULAR REPLACEMENT / Resolution: 2.39 Å | ||||||
 Authors Authors | Wu, Z.B. / Liu, K. / Min, J.R. | ||||||
 Citation Citation |  Journal: J.Mol.Biol. / Year: 2022 Journal: J.Mol.Biol. / Year: 2022Title: Family-wide Characterization of Methylated DNA Binding Ability of Arabidopsis MBDs. Authors: Wu, Z. / Chen, S. / Zhou, M. / Jia, L. / Li, Z. / Zhang, X. / Min, J. / Liu, K. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  7fef.cif.gz 7fef.cif.gz | 41.5 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb7fef.ent.gz pdb7fef.ent.gz | 23.1 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  7fef.json.gz 7fef.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/fe/7fef https://data.pdbj.org/pub/pdb/validation_reports/fe/7fef ftp://data.pdbj.org/pub/pdb/validation_reports/fe/7fef ftp://data.pdbj.org/pub/pdb/validation_reports/fe/7fef | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  7feoC  2yk8  6c1aS S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 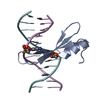
| ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||||
| Unit cell |
|
- Components
Components
| #1: DNA chain | Mass: 3677.419 Da / Num. of mol.: 2 / Source method: obtained synthetically / Source: (synth.) synthetic construct (others) #2: Protein | | Mass: 8210.141 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   #3: Water | ChemComp-HOH / | Has ligand of interest | Y | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.53 Å3/Da / Density % sol: 51.4 % |
|---|---|
| Crystal grow | Temperature: 291 K / Method: vapor diffusion, sitting drop / pH: 6.5 / Details: 0.1M Bis-tris pH 6.5, 25% PEG 3350 |
-Data collection
| Diffraction | Mean temperature: 100 K / Serial crystal experiment: N |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  SSRF SSRF  / Beamline: BL19U1 / Wavelength: 0.9785 Å / Beamline: BL19U1 / Wavelength: 0.9785 Å |
| Detector | Type: DECTRIS EIGER X 16M / Detector: PIXEL / Date: Jul 6, 2021 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.9785 Å / Relative weight: 1 |
| Reflection | Resolution: 2.29→34.91 Å / Num. obs: 6926 / % possible obs: 99.9 % / Redundancy: 10.3 % / Biso Wilson estimate: 75.32 Å2 / CC1/2: 0.989 / Net I/σ(I): 12.4 |
| Reflection shell | Resolution: 2.29→2.37 Å / Num. unique obs: 692 / CC1/2: 0.644 / % possible all: 98.9 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 6c1a, 2yk8 Resolution: 2.39→34.91 Å / Cor.coef. Fo:Fc: 0.957 / Cor.coef. Fo:Fc free: 0.951 / SU ML: 0.21 / Cross valid method: THROUGHOUT / σ(F): 0 / ESU R: 0.353 / ESU R Free: 0.244 / Stereochemistry target values: MAXIMUM LIKELIHOOD Details: HYDROGENS HAVE BEEN DADED IN THE RIDING POSITIONS U VALUES : REFINED INDIVIDUALLY
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Solvent model: MASK | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso max: 139.46 Å2 / Biso mean: 69.3496 Å2 / Biso min: 42.86 Å2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: final / Resolution: 2.39→34.91 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 2.39→2.448 Å / Rfactor Rfree error: 0
|
 Movie
Movie Controller
Controller










 PDBj
PDBj







































