+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 7dxh | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of PSII intermediate Psb28-PSII complex | ||||||||||||||||||||||||||||||
 Components Components |
| ||||||||||||||||||||||||||||||
 Keywords Keywords | PLANT PROTEIN / Photosystem II / Cryo-EM / assembly / repair / Psb28 / Tsl0063 | ||||||||||||||||||||||||||||||
| Function / homology |  Function and homology information Function and homology informationphotosystem II oxygen evolving complex / oxygen evolving activity / photosystem II stabilization / photosystem II reaction center / photosystem II / oxidoreductase activity, acting on diphenols and related substances as donors, oxygen as acceptor / photosynthetic electron transport chain / : / response to herbicide / photosystem II ...photosystem II oxygen evolving complex / oxygen evolving activity / photosystem II stabilization / photosystem II reaction center / photosystem II / oxidoreductase activity, acting on diphenols and related substances as donors, oxygen as acceptor / photosynthetic electron transport chain / : / response to herbicide / photosystem II / plasma membrane-derived thylakoid membrane / photosynthetic electron transport in photosystem II / chlorophyll binding / phosphate ion binding / photosynthesis, light reaction / photosynthesis / electron transfer activity / protein stabilization / iron ion binding / heme binding / metal ion binding Similarity search - Function | ||||||||||||||||||||||||||||||
| Biological species |  Thermosynechococcus vulcanus (bacteria) Thermosynechococcus vulcanus (bacteria) | ||||||||||||||||||||||||||||||
| Method | ELECTRON MICROSCOPY / single particle reconstruction / cryo EM / Resolution: 3.14 Å | ||||||||||||||||||||||||||||||
 Authors Authors | Sui, S.F. / Shen, J.R. / Han, G.Y. / Xiao, Y.N. / Huang, G.Q. | ||||||||||||||||||||||||||||||
 Citation Citation |  Journal: Nat Plants / Year: 2021 Journal: Nat Plants / Year: 2021Title: Structural insights into cyanobacterial photosystem II intermediates associated with Psb28 and Tsl0063. Authors: Yanan Xiao / Guoqiang Huang / Xin You / Qingjun Zhu / Wenda Wang / Tingyun Kuang / Guangye Han / Sen-Fang Sui / Jian-Ren Shen /   Abstract: Photosystem II (PSII) is a multisubunit pigment-protein complex and catalyses light-induced water oxidation, leading to the conversion of light energy into chemical energy and the release of dioxygen. ...Photosystem II (PSII) is a multisubunit pigment-protein complex and catalyses light-induced water oxidation, leading to the conversion of light energy into chemical energy and the release of dioxygen. We analysed the structures of two Psb28-bound PSII intermediates, Psb28-RC47 and Psb28-PSII, purified from a psbV-deletion strain of the thermophilic cyanobacterium Thermosynechococcus vulcanus, using cryo-electron microscopy. Both Psb28-RC47 and Psb28-PSII bind one Psb28, one Tsl0063 and an unknown subunit. Psb28 is located at the cytoplasmic surface of PSII and interacts with D1, D2 and CP47, whereas Tsl0063 is a transmembrane subunit and binds at the side of CP47/PsbH. Substantial structural perturbations are observed at the acceptor side, which result in conformational changes of the quinone (Q) and non-haem iron binding sites and thus may protect PSII from photodamage during assembly. These results provide a solid structural basis for understanding the assembly process of native PSII. | ||||||||||||||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  7dxh.cif.gz 7dxh.cif.gz | 476.2 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb7dxh.ent.gz pdb7dxh.ent.gz | 390.3 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  7dxh.json.gz 7dxh.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/dx/7dxh https://data.pdbj.org/pub/pdb/validation_reports/dx/7dxh ftp://data.pdbj.org/pub/pdb/validation_reports/dx/7dxh ftp://data.pdbj.org/pub/pdb/validation_reports/dx/7dxh | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  30909MC  7dxaC M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
-Photosystem II ... , 14 types, 14 molecules Aabdhilmtxckzy
| #1: Protein | Mass: 13188.769 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  Thermosynechococcus vulcanus (bacteria) / References: UniProt: Q8DLJ8 Thermosynechococcus vulcanus (bacteria) / References: UniProt: Q8DLJ8 |
|---|---|
| #3: Protein | Mass: 39792.367 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  Thermosynechococcus vulcanus (bacteria) / References: UniProt: P51765 Thermosynechococcus vulcanus (bacteria) / References: UniProt: P51765 |
| #4: Protein | Mass: 56068.742 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  Thermosynechococcus vulcanus (bacteria) / References: UniProt: D0VWR1 Thermosynechococcus vulcanus (bacteria) / References: UniProt: D0VWR1 |
| #5: Protein | Mass: 38404.949 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  Thermosynechococcus vulcanus (bacteria) / References: UniProt: D0VWR8 Thermosynechococcus vulcanus (bacteria) / References: UniProt: D0VWR8 |
| #8: Protein | Mass: 7227.559 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  Thermosynechococcus vulcanus (bacteria) / References: UniProt: P19052 Thermosynechococcus vulcanus (bacteria) / References: UniProt: P19052 |
| #9: Protein/peptide | Mass: 4410.245 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  Thermosynechococcus vulcanus (bacteria) / References: UniProt: P12240 Thermosynechococcus vulcanus (bacteria) / References: UniProt: P12240 |
| #10: Protein/peptide | Mass: 4299.044 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  Thermosynechococcus vulcanus (bacteria) / References: UniProt: P12241 Thermosynechococcus vulcanus (bacteria) / References: UniProt: P12241 |
| #11: Protein/peptide | Mass: 4015.688 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  Thermosynechococcus vulcanus (bacteria) / References: UniProt: P12312 Thermosynechococcus vulcanus (bacteria) / References: UniProt: P12312 |
| #12: Protein/peptide | Mass: 3878.728 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  Thermosynechococcus vulcanus (bacteria) / References: UniProt: P12313 Thermosynechococcus vulcanus (bacteria) / References: UniProt: P12313 |
| #13: Protein/peptide | Mass: 4191.030 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  Thermosynechococcus vulcanus (bacteria) / References: UniProt: D0VWR4 Thermosynechococcus vulcanus (bacteria) / References: UniProt: D0VWR4 |
| #14: Protein | Mass: 49207.250 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  Thermosynechococcus vulcanus (bacteria) / References: UniProt: D0VWR7 Thermosynechococcus vulcanus (bacteria) / References: UniProt: D0VWR7 |
| #15: Protein/peptide | Mass: 5028.083 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  Thermosynechococcus vulcanus (bacteria) / References: UniProt: Q9F1K9 Thermosynechococcus vulcanus (bacteria) / References: UniProt: Q9F1K9 |
| #16: Protein | Mass: 6766.187 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  Thermosynechococcus vulcanus (bacteria) / References: UniProt: D0VWR5 Thermosynechococcus vulcanus (bacteria) / References: UniProt: D0VWR5 |
| #17: Protein/peptide | Mass: 3228.035 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  Thermosynechococcus vulcanus (bacteria) / References: UniProt: D0VWR3 Thermosynechococcus vulcanus (bacteria) / References: UniProt: D0VWR3 |
-Cytochrome b559 subunit ... , 2 types, 2 molecules ef
| #6: Protein | Mass: 9580.840 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  Thermosynechococcus vulcanus (bacteria) / References: UniProt: P12238 Thermosynechococcus vulcanus (bacteria) / References: UniProt: P12238 |
|---|---|
| #7: Protein/peptide | Mass: 5067.900 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  Thermosynechococcus vulcanus (bacteria) / References: UniProt: P12239 Thermosynechococcus vulcanus (bacteria) / References: UniProt: P12239 |
-Protein / Protein/peptide , 2 types, 2 molecules BC
| #18: Protein/peptide | Mass: 1652.805 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  Thermosynechococcus vulcanus (bacteria) Thermosynechococcus vulcanus (bacteria) |
|---|---|
| #2: Protein | Mass: 5943.893 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  Thermosynechococcus vulcanus (bacteria) / References: UniProt: Q8DMP8 Thermosynechococcus vulcanus (bacteria) / References: UniProt: Q8DMP8 |
-Sugars , 3 types, 8 molecules 

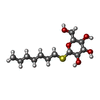


| #24: Sugar | ChemComp-DGD / #26: Sugar | #31: Sugar | ChemComp-HTG / | |
|---|
-Non-polymers , 10 types, 61 molecules 















| #19: Chemical | ChemComp-MGE / ( #20: Chemical | #21: Chemical | ChemComp-CLA / #22: Chemical | ChemComp-BCR / #23: Chemical | #25: Chemical | #27: Chemical | ChemComp-FE2 / | #28: Chemical | ChemComp-PQ9 / | #29: Chemical | Mass: 294.408 Da / Num. of mol.: 2 / Source method: obtained synthetically #30: Chemical | ChemComp-HEM / | |
|---|
-Details
| Has ligand of interest | N |
|---|---|
| Has protein modification | N |
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method: single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: Cryo-EM structure of PSII intermediate Psb28-PSII complex. Type: COMPLEX / Entity ID: #1-#11, #13-#18 / Source: NATURAL |
|---|---|
| Source (natural) | Organism:  Thermosynechococcus vulcanus (bacteria) Thermosynechococcus vulcanus (bacteria) |
| Buffer solution | pH: 6.5 |
| Specimen | Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: YES |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TITAN KRIOS |
| Electron gun | Electron source:  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD |
| Image recording | Electron dose: 50 e/Å2 / Film or detector model: GATAN K2 SUMMIT (4k x 4k) |
- Processing
Processing
| Software | Name: PHENIX / Version: 1.17.1_3660: / Classification: refinement | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EM software | Name: PHENIX / Category: model refinement | ||||||||||||||||||||||||
| CTF correction | Type: NONE | ||||||||||||||||||||||||
| 3D reconstruction | Resolution: 3.14 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 194738 / Symmetry type: POINT | ||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller












 PDBj
PDBj





















