+ データを開く
データを開く
- 基本情報
基本情報
| 登録情報 | データベース: PDB / ID: 7df3 | ||||||
|---|---|---|---|---|---|---|---|
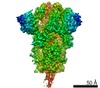
| タイトル | SARS-CoV-2 S trimer, S-closed | ||||||
 要素 要素 | Spike glycoprotein | ||||||
 キーワード キーワード | VIRAL PROTEIN / Spike glycoprotein / coronavirus / SARS-CoV-2 virus / closed state | ||||||
| 機能・相同性 |  機能・相同性情報 機能・相同性情報symbiont-mediated disruption of host tissue / Maturation of spike protein / Translation of Structural Proteins / Virion Assembly and Release / host cell surface / host extracellular space / viral translation / symbiont-mediated-mediated suppression of host tetherin activity / Induction of Cell-Cell Fusion / structural constituent of virion ...symbiont-mediated disruption of host tissue / Maturation of spike protein / Translation of Structural Proteins / Virion Assembly and Release / host cell surface / host extracellular space / viral translation / symbiont-mediated-mediated suppression of host tetherin activity / Induction of Cell-Cell Fusion / structural constituent of virion / entry receptor-mediated virion attachment to host cell / membrane fusion / Attachment and Entry / host cell endoplasmic reticulum-Golgi intermediate compartment membrane / positive regulation of viral entry into host cell / receptor-mediated virion attachment to host cell / host cell surface receptor binding / symbiont-mediated suppression of host innate immune response / receptor ligand activity / endocytosis involved in viral entry into host cell / fusion of virus membrane with host plasma membrane / fusion of virus membrane with host endosome membrane / viral envelope / symbiont entry into host cell / virion attachment to host cell / SARS-CoV-2 activates/modulates innate and adaptive immune responses / host cell plasma membrane / virion membrane / identical protein binding / membrane / plasma membrane 類似検索 - 分子機能 | ||||||
| 生物種 |  | ||||||
| 手法 | 電子顕微鏡法 / 単粒子再構成法 / クライオ電子顕微鏡法 / 解像度: 2.7 Å | ||||||
 データ登録者 データ登録者 | Cong, X. / Yao, C. | ||||||
| 資金援助 |  中国, 1件 中国, 1件
| ||||||
 引用 引用 |  ジャーナル: Sci Adv / 年: 2021 ジャーナル: Sci Adv / 年: 2021タイトル: Conformational dynamics of SARS-CoV-2 trimeric spike glycoprotein in complex with receptor ACE2 revealed by cryo-EM. 著者: Cong Xu / Yanxing Wang / Caixuan Liu / Chao Zhang / Wenyu Han / Xiaoyu Hong / Yifan Wang / Qin Hong / Shutian Wang / Qiaoyu Zhao / Yalei Wang / Yong Yang / Kaijian Chen / Wei Zheng / ...著者: Cong Xu / Yanxing Wang / Caixuan Liu / Chao Zhang / Wenyu Han / Xiaoyu Hong / Yifan Wang / Qin Hong / Shutian Wang / Qiaoyu Zhao / Yalei Wang / Yong Yang / Kaijian Chen / Wei Zheng / Liangliang Kong / Fangfang Wang / Qinyu Zuo / Zhong Huang / Yao Cong /  要旨: The recent outbreaks of SARS-CoV-2 pose a global health emergency. The SARS-CoV-2 trimeric spike (S) glycoprotein interacts with the human ACE2 receptor to mediate viral entry into host cells. We ...The recent outbreaks of SARS-CoV-2 pose a global health emergency. The SARS-CoV-2 trimeric spike (S) glycoprotein interacts with the human ACE2 receptor to mediate viral entry into host cells. We report the cryo-EM structures of a tightly closed SARS-CoV-2 S trimer with packed fusion peptide and an ACE2-bound S trimer at 2.7- and 3.8-Å resolution, respectively. Accompanying ACE2 binding to the up receptor-binding domain (RBD), the associated ACE2-RBD exhibits continuous swing motions. Notably, the SARS-CoV-2 S trimer appears much more sensitive to the ACE2 receptor than the SARS-CoV S trimer regarding receptor-triggered transformation from the closed prefusion state to the fusion-prone open state, potentially contributing to the superior infectivity of SARS-CoV-2. We defined the RBD T470-T478 loop and Y505 as viral determinants for specific recognition of SARS-CoV-2 RBD by ACE2. Our findings depict the mechanism of ACE2-induced S trimer conformational transitions from the ground prefusion state toward the postfusion state, facilitating development of anti-SARS-CoV-2 vaccines and therapeutics. | ||||||
| 履歴 |
|
- 構造の表示
構造の表示
| ムービー |
 ムービービューア ムービービューア |
|---|---|
| 構造ビューア | 分子:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
- ダウンロードとリンク
ダウンロードとリンク
- ダウンロード
ダウンロード
| PDBx/mmCIF形式 |  7df3.cif.gz 7df3.cif.gz | 635.4 KB | 表示 |  PDBx/mmCIF形式 PDBx/mmCIF形式 |
|---|---|---|---|---|
| PDB形式 |  pdb7df3.ent.gz pdb7df3.ent.gz | 521.4 KB | 表示 |  PDB形式 PDB形式 |
| PDBx/mmJSON形式 |  7df3.json.gz 7df3.json.gz | ツリー表示 |  PDBx/mmJSON形式 PDBx/mmJSON形式 | |
| その他 |  その他のダウンロード その他のダウンロード |
-検証レポート
| 文書・要旨 |  7df3_validation.pdf.gz 7df3_validation.pdf.gz | 2 MB | 表示 |  wwPDB検証レポート wwPDB検証レポート |
|---|---|---|---|---|
| 文書・詳細版 |  7df3_full_validation.pdf.gz 7df3_full_validation.pdf.gz | 2.1 MB | 表示 | |
| XML形式データ |  7df3_validation.xml.gz 7df3_validation.xml.gz | 97.7 KB | 表示 | |
| CIF形式データ |  7df3_validation.cif.gz 7df3_validation.cif.gz | 144.1 KB | 表示 | |
| アーカイブディレクトリ |  https://data.pdbj.org/pub/pdb/validation_reports/df/7df3 https://data.pdbj.org/pub/pdb/validation_reports/df/7df3 ftp://data.pdbj.org/pub/pdb/validation_reports/df/7df3 ftp://data.pdbj.org/pub/pdb/validation_reports/df/7df3 | HTTPS FTP |
-関連構造データ
- リンク
リンク
- 集合体
集合体
| 登録構造単位 | 
|
|---|---|
| 1 |
|
- 要素
要素
| #1: タンパク質 | 分子量: 140055.906 Da / 分子数: 3 / 由来タイプ: 組換発現 由来: (組換発現)  遺伝子: S, 2 / 細胞株 (発現宿主): HEK293F / 発現宿主:  Homo sapiens (ヒト) / 参照: UniProt: P0DTC2 Homo sapiens (ヒト) / 参照: UniProt: P0DTC2#2: 多糖 | 2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose #3: 糖 | ChemComp-NAG / 研究の焦点であるリガンドがあるか | N | Has protein modification | Y | |
|---|
-実験情報
-実験
| 実験 | 手法: 電子顕微鏡法 |
|---|---|
| EM実験 | 試料の集合状態: PARTICLE / 3次元再構成法: 単粒子再構成法 |
- 試料調製
試料調製
| 構成要素 | 名称: SARS-CoV-2 spike glycoprotein trimer / タイプ: COMPLEX / Entity ID: #1 / 由来: RECOMBINANT |
|---|---|
| 由来(天然) | 生物種:  |
| 由来(組換発現) | 生物種:  Homo sapiens (ヒト) / 株: HEK293F Homo sapiens (ヒト) / 株: HEK293F |
| 緩衝液 | pH: 7.5 |
| 試料 | 包埋: NO / シャドウイング: NO / 染色: NO / 凍結: YES |
| 急速凍結 | 凍結剤: ETHANE |
- 電子顕微鏡撮影
電子顕微鏡撮影
| 実験機器 |  モデル: Titan Krios / 画像提供: FEI Company |
|---|---|
| 顕微鏡 | モデル: FEI TITAN KRIOS |
| 電子銃 | 電子線源:  FIELD EMISSION GUN / 加速電圧: 300 kV / 照射モード: FLOOD BEAM FIELD EMISSION GUN / 加速電圧: 300 kV / 照射モード: FLOOD BEAM |
| 電子レンズ | モード: BRIGHT FIELD |
| 撮影 | 電子線照射量: 50 e/Å2 フィルム・検出器のモデル: GATAN K2 SUMMIT (4k x 4k) |
- 解析
解析
| CTF補正 | タイプ: PHASE FLIPPING AND AMPLITUDE CORRECTION |
|---|---|
| 3次元再構成 | 解像度: 2.7 Å / 解像度の算出法: FSC 0.143 CUT-OFF / 粒子像の数: 142345 / 対称性のタイプ: POINT |
 ムービー
ムービー コントローラー
コントローラー

















 PDBj
PDBj





