+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 7b87 | ||||||
|---|---|---|---|---|---|---|---|
| Title | Notum-Fragment074 | ||||||
 Components Components | Palmitoleoyl-protein carboxylesterase NOTUM | ||||||
 Keywords Keywords | HYDROLASE / Notum Fragment | ||||||
| Function / homology |  Function and homology information Function and homology informationprotein-containing complex destabilizing activity / [Wnt protein] O-palmitoleoyl-L-serine hydrolase / protein depalmitoleylation / palmitoleyl hydrolase activity / Release of Hh-Np from the secreting cell / C-type glycerophospholipase activity / regulation of bone mineralization / negative regulation of Wnt signaling pathway / Post-translational protein phosphorylation / negative regulation of canonical Wnt signaling pathway ...protein-containing complex destabilizing activity / [Wnt protein] O-palmitoleoyl-L-serine hydrolase / protein depalmitoleylation / palmitoleyl hydrolase activity / Release of Hh-Np from the secreting cell / C-type glycerophospholipase activity / regulation of bone mineralization / negative regulation of Wnt signaling pathway / Post-translational protein phosphorylation / negative regulation of canonical Wnt signaling pathway / bone development / Regulation of Insulin-like Growth Factor (IGF) transport and uptake by Insulin-like Growth Factor Binding Proteins (IGFBPs) / Wnt signaling pathway / endoplasmic reticulum lumen / extracellular region Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.58 Å MOLECULAR REPLACEMENT / Resolution: 1.58 Å | ||||||
 Authors Authors | Zhao, Y. / Jonees, E.Y. | ||||||
| Funding support |  United Kingdom, 1items United Kingdom, 1items
| ||||||
 Citation Citation |  Journal: Acs Chem Neurosci / Year: 2022 Journal: Acs Chem Neurosci / Year: 2022Title: Structural Analysis and Development of Notum Fragment Screening Hits. Authors: Zhao, Y. / Mahy, W. / Willis, N.J. / Woodward, H.L. / Steadman, D. / Bayle, E.D. / Atkinson, B.N. / Sipthorp, J. / Vecchia, L. / Ruza, R.R. / Harlos, K. / Jeganathan, F. / Constantinou, S. / ...Authors: Zhao, Y. / Mahy, W. / Willis, N.J. / Woodward, H.L. / Steadman, D. / Bayle, E.D. / Atkinson, B.N. / Sipthorp, J. / Vecchia, L. / Ruza, R.R. / Harlos, K. / Jeganathan, F. / Constantinou, S. / Costa, A. / Kjaer, S. / Bictash, M. / Salinas, P.C. / Whiting, P. / Vincent, J.P. / Fish, P.V. / Jones, E.Y. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  7b87.cif.gz 7b87.cif.gz | 163.7 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb7b87.ent.gz pdb7b87.ent.gz | 126 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  7b87.json.gz 7b87.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/b8/7b87 https://data.pdbj.org/pub/pdb/validation_reports/b8/7b87 ftp://data.pdbj.org/pub/pdb/validation_reports/b8/7b87 ftp://data.pdbj.org/pub/pdb/validation_reports/b8/7b87 | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  7b4xC  7b84C  7b86C  7b89C  7b8cC  7b8dC  7b8fC  7b8gC  7b8jC  7b8kC  7b8lC  7b8mC  7b8nC  7b8oC  7b8uC  7b8xC  7b8yC  7b8zC  7b98C  7b99C  7b9dC  7b9iC  7b9nC  7b9uC  7ba1C  7bacC  7bapC  7bc8C  7bc9C  7bccC  7bcdC  7bceC  7bcfC  7bchC  7bciC  7bckC  7bclC  7bd2C  7bd3C  7bd4C  7bd5C  7bd6C  7bd8C  7bd9C  7bdaC  7bdbC  7bdcC  7bddC  7bdfC  7bdgC  7bdhC  4uzqS C: citing same article ( S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
-Protein / Sugars , 2 types, 2 molecules A

| #1: Protein | Mass: 43567.148 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: NOTUM, OK/SW-CL.30 / Production host: Homo sapiens (human) / Gene: NOTUM, OK/SW-CL.30 / Production host:  Homo sapiens (human) Homo sapiens (human)References: UniProt: Q6P988, [Wnt protein] O-palmitoleoyl-L-serine hydrolase |
|---|---|
| #5: Sugar | ChemComp-NAG / |
-Non-polymers , 5 types, 131 molecules 


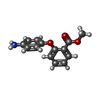





| #2: Chemical | | #3: Chemical | ChemComp-EDO / #4: Chemical | #6: Chemical | ChemComp-JFV / | #7: Water | ChemComp-HOH / | |
|---|
-Details
| Has ligand of interest | Y |
|---|---|
| Has protein modification | Y |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 1.98 Å3/Da / Density % sol: 37.72 % |
|---|---|
| Crystal grow | Temperature: 296 K / Method: evaporation Details: 1.5 M Ammonium Sulphate 0.1 M Sodium Citrate, pH4.2 |
-Data collection
| Diffraction | Mean temperature: 100 K / Serial crystal experiment: N |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  Diamond Diamond  / Beamline: I04-1 / Wavelength: 0.9282 Å / Beamline: I04-1 / Wavelength: 0.9282 Å |
| Detector | Type: DECTRIS PILATUS3 S 6M / Detector: PIXEL / Date: Feb 2, 2017 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.9282 Å / Relative weight: 1 |
| Reflection | Resolution: 1.58→73.16 Å / Num. obs: 47981 / % possible obs: 100 % / Redundancy: 6.8 % / CC1/2: 1 / Net I/σ(I): 6.8 |
| Reflection shell | Resolution: 1.58→1.61 Å / Num. unique obs: 2329 / CC1/2: 0.536 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 4UZQ Resolution: 1.58→53.58 Å / SU ML: 0.24 / Cross valid method: THROUGHOUT / σ(F): 1.33 / Phase error: 27.7 / Stereochemistry target values: ML
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å / Solvent model: FLAT BULK SOLVENT MODEL | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso max: 84.59 Å2 / Biso mean: 23.5545 Å2 / Biso min: 11.15 Å2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: final / Resolution: 1.58→53.58 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Refine-ID: X-RAY DIFFRACTION / Rfactor Rfree error: 0 / Total num. of bins used: 17
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Origin x: 4.5832 Å / Origin y: -1.3677 Å / Origin z: -2.6569 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group |
|
 Movie
Movie Controller
Controller
















 PDBj
PDBj




