[English] 日本語
 Yorodumi
Yorodumi- PDB-4uzq: STRUCTURE OF THE WNT DEACYLASE NOTUM IN COMPLEX WITH O-PALMITOLEO... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 4uzq | ||||||
|---|---|---|---|---|---|---|---|
| Title | STRUCTURE OF THE WNT DEACYLASE NOTUM IN COMPLEX WITH O-PALMITOLEOYL SERINE - CRYSTAL FORM IX - 1.5A | ||||||
 Components Components |
| ||||||
 Keywords Keywords | HYDROLASE / ESTERASE / EXTRACELLULAR / ALPHA/BETA HYDROLASE | ||||||
| Function / homology |  Function and homology information Function and homology informationpostsynapse assembly / positive regulation of excitatory synapse assembly / positive regulation of protein localization to presynapse / skeletal muscle satellite cell activation / regulation of axon diameter / asymmetric protein localization involved in cell fate determination / lens fiber cell development / cerebellar granule cell differentiation / oviduct development / WNT ligand biogenesis and trafficking ...postsynapse assembly / positive regulation of excitatory synapse assembly / positive regulation of protein localization to presynapse / skeletal muscle satellite cell activation / regulation of axon diameter / asymmetric protein localization involved in cell fate determination / lens fiber cell development / cerebellar granule cell differentiation / oviduct development / WNT ligand biogenesis and trafficking / synaptic vesicle recycling / central nervous system vasculogenesis / cell proliferation in forebrain / excitatory synapse assembly / uterus morphogenesis / embryonic axis specification / secondary palate development / presynapse assembly / somatic stem cell division / skeletal muscle satellite cell maintenance involved in skeletal muscle regeneration / stem cell development / sex differentiation / protein-containing complex destabilizing activity / [Wnt protein] O-palmitoleoyl-L-serine hydrolase / protein depalmitoleylation / palmitoleyl hydrolase activity / positive regulation of epithelial cell proliferation involved in wound healing / Release of Hh-Np from the secreting cell / C-type glycerophospholipase activity / regulation of bone mineralization / establishment of blood-brain barrier / dendritic spine morphogenesis / frizzled binding / dorsal/ventral pattern formation / embryonic forelimb morphogenesis / Class B/2 (Secretin family receptors) / neurotransmitter secretion / regulation of postsynapse organization / wound healing, spreading of epidermal cells / embryonic hindlimb morphogenesis / positive regulation of synapse assembly / Wnt signaling pathway, planar cell polarity pathway / embryonic digit morphogenesis / cartilage condensation / regulation of synaptic vesicle exocytosis / establishment of cell polarity / positive regulation of protein metabolic process / negative regulation of Wnt signaling pathway / somatic stem cell population maintenance / cell fate commitment / chondrocyte differentiation / positive regulation of excitatory postsynaptic potential / canonical Wnt signaling pathway / regulation of presynapse assembly / cellular response to transforming growth factor beta stimulus / extracellular matrix / positive regulation of endothelial cell migration / axonogenesis / cytokine activity / Post-translational protein phosphorylation / positive regulation of JNK cascade / negative regulation of canonical Wnt signaling pathway / bone development / negative regulation of neurogenesis / Golgi lumen / response to estrogen / Schaffer collateral - CA1 synapse / Regulation of Insulin-like Growth Factor (IGF) transport and uptake by Insulin-like Growth Factor Binding Proteins (IGFBPs) / Wnt signaling pathway / neuron differentiation / endocytic vesicle membrane / response to estradiol / presynapse / angiogenesis / receptor ligand activity / endoplasmic reticulum lumen / signaling receptor binding / apoptotic process / positive regulation of gene expression / negative regulation of apoptotic process / positive regulation of DNA-templated transcription / glutamatergic synapse / cell surface / positive regulation of transcription by RNA polymerase II / extracellular space / extracellular exosome / extracellular region / plasma membrane Similarity search - Function | ||||||
| Biological species |  HOMO SAPIENS (human) HOMO SAPIENS (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / OTHER / Resolution: 1.5 Å SYNCHROTRON / OTHER / Resolution: 1.5 Å | ||||||
 Authors Authors | Zebisch, M. / Jones, E.Y. | ||||||
 Citation Citation |  Journal: Nature / Year: 2015 Journal: Nature / Year: 2015Title: Notum Deacylates Wnt Proteins to Suppress Signalling Activity. Authors: Kakugawa, S. / Langton, P.F. / Zebisch, M. / Howell, S.A. / Chang, T. / Liu, Y. / Feizi, T. / Bineva, G. / O'Reilly, N. / Snijders, A.P. / Jones, E.Y. / Vincent, J. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  4uzq.cif.gz 4uzq.cif.gz | 177.1 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb4uzq.ent.gz pdb4uzq.ent.gz | 138.1 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  4uzq.json.gz 4uzq.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/uz/4uzq https://data.pdbj.org/pub/pdb/validation_reports/uz/4uzq ftp://data.pdbj.org/pub/pdb/validation_reports/uz/4uzq ftp://data.pdbj.org/pub/pdb/validation_reports/uz/4uzq | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  4uyuC  4uywC  4uyzC  4uz1C  4uz5C  4uz6C  4uz7C  4uz9C  4uzaC  4uzjC  4uzkC  4uzlC  4wbhC C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| Unit cell |
| ||||||||
| Components on special symmetry positions |
|
- Components
Components
-Protein / Protein/peptide / Sugars , 3 types, 3 molecules AB

| #1: Protein | Mass: 43974.547 Da / Num. of mol.: 1 / Fragment: RESIDUES 81-451 / Mutation: YES Source method: isolated from a genetically manipulated source Details: GLYCOSYLATED AT N96 / Source: (gene. exp.)  HOMO SAPIENS (human) / Plasmid: PHLSEC / Cell line (production host): HEK293T / Production host: HOMO SAPIENS (human) / Plasmid: PHLSEC / Cell line (production host): HEK293T / Production host:  HOMO SAPIENS (human) / References: UniProt: Q6P988, carboxylesterase HOMO SAPIENS (human) / References: UniProt: Q6P988, carboxylesterase |
|---|---|
| #2: Protein/peptide | Mass: 749.838 Da / Num. of mol.: 1 / Fragment: RESIDUES 202-209 / Source method: obtained synthetically / Source: (synth.)  HOMO SAPIENS (human) / References: UniProt: O00755 HOMO SAPIENS (human) / References: UniProt: O00755 |
| #3: Sugar | ChemComp-NAG / |
-Non-polymers , 4 types, 311 molecules 

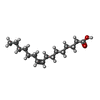




| #4: Chemical | | #5: Chemical | #6: Chemical | ChemComp-PAM / | #7: Water | ChemComp-HOH / | |
|---|
-Details
| Has protein modification | Y | ||
|---|---|---|---|
| Nonpolymer details | GAMMA-PALMITOLEO| Sequence details | C330S ENGINEERED | |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 4 X-RAY DIFFRACTION / Number of used crystals: 4 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.4 Å3/Da / Density % sol: 49 % / Description: NONE |
|---|---|
| Crystal grow | pH: 5 / Details: 3.0M NACL, 0.1 M CITRATE PH 5.0 |
-Data collection
| Diffraction | Mean temperature: 100 K | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  Diamond Diamond  / Beamline: I04 / Wavelength: 1.00,1.07 / Beamline: I04 / Wavelength: 1.00,1.07 | |||||||||
| Detector | Type: DECTRIS PILATUS 6M / Detector: PIXEL / Date: Jul 13, 2014 | |||||||||
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray | |||||||||
| Radiation wavelength |
| |||||||||
| Reflection | Resolution: 1.5→60 Å / Num. obs: 64352 / % possible obs: 96.4 % / Observed criterion σ(I): -3 / Redundancy: 3.4 % / Rmerge(I) obs: 0.1 / Net I/σ(I): 11.6 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure: OTHER Starting model: NONE Resolution: 1.5→60.44 Å / Cor.coef. Fo:Fc: 0.978 / Cor.coef. Fo:Fc free: 0.962 / SU B: 2.98 / SU ML: 0.047 / Cross valid method: THROUGHOUT / ESU R: 0.061 / ESU R Free: 0.062 / Stereochemistry target values: MAXIMUM LIKELIHOOD Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS. U VALUES REFINED INDIVIDUALLY
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.2 Å / Solvent model: MASK | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 17.448 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.5→60.44 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller












 PDBj
PDBj


