+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 6y81 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Fragment KCL_1088 in complex with MAP kinase p38-alpha | |||||||||
 Components Components | Mitogen-activated protein kinase 14 | |||||||||
 Keywords Keywords | TRANSFERASE / FBDD / FRAGMENT BASED DRUG DESIGN / P38 / MAPK14 / KINASE | |||||||||
| Function / homology |  Function and homology information Function and homology informationp38MAPK events / Activation of the AP-1 family of transcription factors / Platelet sensitization by LDL / RHO GTPases Activate NADPH Oxidases / ERK/MAPK targets / myoblast differentiation involved in skeletal muscle regeneration / NLRP1 inflammasome complex assembly / Regulation of MITF-M-dependent genes involved in pigmentation / activated TAK1 mediates p38 MAPK activation / NOD1/2 Signaling Pathway ...p38MAPK events / Activation of the AP-1 family of transcription factors / Platelet sensitization by LDL / RHO GTPases Activate NADPH Oxidases / ERK/MAPK targets / myoblast differentiation involved in skeletal muscle regeneration / NLRP1 inflammasome complex assembly / Regulation of MITF-M-dependent genes involved in pigmentation / activated TAK1 mediates p38 MAPK activation / NOD1/2 Signaling Pathway / Oxidative Stress Induced Senescence / ADP signalling through P2Y purinoceptor 1 / Regulation of TP53 Activity through Phosphorylation / Myogenesis / VEGFA-VEGFR2 Pathway / regulation of synaptic membrane adhesion / stress-induced premature senescence / cell surface receptor protein serine/threonine kinase signaling pathway / stress-activated protein kinase signaling cascade / positive regulation of myoblast fusion / cellular response to UV-B / cartilage condensation / mitogen-activated protein kinase p38 binding / positive regulation of myotube differentiation / NFAT protein binding / regulation of cytokine production involved in inflammatory response / p38MAPK cascade / cellular response to lipoteichoic acid / response to muramyl dipeptide / response to dietary excess / fatty acid oxidation / pyroptotic inflammatory response / MAP kinase activity / cellular response to vascular endothelial growth factor stimulus / regulation of ossification / mitogen-activated protein kinase / chondrocyte differentiation / vascular endothelial growth factor receptor signaling pathway / negative regulation of hippo signaling / positive regulation of myoblast differentiation / skeletal muscle tissue development / stress-activated MAPK cascade / positive regulation of cardiac muscle cell proliferation / signal transduction in response to DNA damage / positive regulation of brown fat cell differentiation / response to muscle stretch / striated muscle cell differentiation / Neutrophil degranulation / positive regulation of interleukin-12 production / osteoclast differentiation / positive regulation of erythrocyte differentiation / lipopolysaccharide-mediated signaling pathway / placenta development / DNA damage checkpoint signaling / tumor necrosis factor-mediated signaling pathway / cellular response to ionizing radiation / positive regulation of D-glucose import across plasma membrane / protein maturation / negative regulation of canonical Wnt signaling pathway / bone development / positive regulation of protein import into nucleus / cellular response to virus / response to insulin / positive regulation of reactive oxygen species metabolic process / glucose metabolic process / cell morphogenesis / spindle pole / osteoblast differentiation / kinase activity / cellular response to tumor necrosis factor / MAPK cascade / cellular response to lipopolysaccharide / angiogenesis / protein phosphatase binding / response to lipopolysaccharide / protein kinase activity / intracellular signal transduction / nuclear speck / protein serine kinase activity / protein serine/threonine kinase activity / apoptotic process / DNA damage response / positive regulation of gene expression / regulation of transcription by RNA polymerase II / regulation of DNA-templated transcription / glutamatergic synapse / enzyme binding / positive regulation of transcription by RNA polymerase II / mitochondrion / nucleoplasm / ATP binding / nucleus / cytoplasm / cytosol Similarity search - Function | |||||||||
| Biological species |  | |||||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.54 Å MOLECULAR REPLACEMENT / Resolution: 1.54 Å | |||||||||
 Authors Authors | De Nicola, G.F. / Nichols, C.E. | |||||||||
| Funding support |  United Kingdom, 2items United Kingdom, 2items
| |||||||||
 Citation Citation |  Journal: J.Med.Chem. / Year: 2020 Journal: J.Med.Chem. / Year: 2020Title: Mining the PDB for Tractable Cases Where X-ray Crystallography Combined with Fragment Screens Can Be Used to Systematically Design Protein-Protein Inhibitors: Two Test Cases Illustrated by IL1 ...Title: Mining the PDB for Tractable Cases Where X-ray Crystallography Combined with Fragment Screens Can Be Used to Systematically Design Protein-Protein Inhibitors: Two Test Cases Illustrated by IL1 beta-IL1R and p38 alpha-TAB1 Complexes. Authors: Nichols, C. / Ng, J. / Keshu, A. / Kelly, G. / Conte, M.R. / Marber, M.S. / Fraternali, F. / De Nicola, G.F. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  6y81.cif.gz 6y81.cif.gz | 104.2 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb6y81.ent.gz pdb6y81.ent.gz | 70.3 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  6y81.json.gz 6y81.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/y8/6y81 https://data.pdbj.org/pub/pdb/validation_reports/y8/6y81 ftp://data.pdbj.org/pub/pdb/validation_reports/y8/6y81 ftp://data.pdbj.org/pub/pdb/validation_reports/y8/6y81 | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  5r85C  5r86C  5r87C  5r88C  5r89C  5r8aC  5r8bC  5r8cC  5r8dC  5r8eC  5r8fC  5r8gC  5r8hC  5r8iC  5r8jC  5r8kC  5r8lC  5r8mC  5r8nC  5r8oC  5r8pC  5r8qC  5r8uC  5r8vC  5r8wC  5r8xC  5r8yC  5r8zC  5r90C  5r91C  5r92C  5r93C  5r94C  5r95C  5r96C  5r97C  5r98C  5r99C  5r9aC  5r9bC  5r9cC  5r9dC  5r9eC  5r9fC  5r9gC  5r9hC  5r9iC  5r9jC  5r9kC  5r9lC  5r9mC  5r9nC  5r9oC  5r9pC  5r9qC  5r9rC  5r9sC  5r9tC  5r9uC  5r9vC  5r9wC  5r9xC  5r9yC  5r9zC  5ra0C  5ra1C  5ra2C  5ra3C  5ra4C  5ra5C  5ra6C  5ra7C  5ra8C  5ra9C  6so1C  6so2C  6so4C  6sodC  6soiC  6sotC  6souC  6sovSC  6sp9C  6splC  6y7wC  6y7xC  6y7yC  6y80C  6ycuC  6ycwC S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||||||
| Unit cell |
| ||||||||||||
| Components on special symmetry positions |
|
- Components
Components
-Protein , 1 types, 1 molecules A
| #1: Protein | Mass: 41322.098 Da / Num. of mol.: 1 / Mutation: C162S Source method: isolated from a genetically manipulated source Source: (gene. exp.)   References: UniProt: P47811, mitogen-activated protein kinase |
|---|
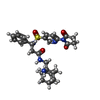
-Non-polymers , 7 types, 235 molecules 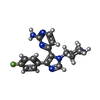











| #2: Chemical | ChemComp-SB4 / | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| #3: Chemical | ChemComp-CA / #4: Chemical | #5: Chemical | ChemComp-DMS / | #6: Chemical | ChemComp-EDO / | #7: Chemical | ChemComp-OG5 / ( | #8: Water | ChemComp-HOH / | |
-Details
| Has ligand of interest | Y |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.61 Å3/Da / Density % sol: 52.85 % |
|---|---|
| Crystal grow | Temperature: 291 K / Method: vapor diffusion, sitting drop Details: 15% PEG400, 5% PEG550-MME, 0.1M CALCIUM ACETATE, 0.1M MES PH6.0 |
-Data collection
| Diffraction | Mean temperature: 100 K / Serial crystal experiment: N |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  Diamond Diamond  / Beamline: I03 / Wavelength: 0.9159 Å / Beamline: I03 / Wavelength: 0.9159 Å |
| Detector | Type: DECTRIS PILATUS 6M-F / Detector: PIXEL / Date: Jul 29, 2018 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.9159 Å / Relative weight: 1 |
| Reflection | Resolution: 1.54→63.88 Å / Num. obs: 64102 / % possible obs: 100 % / Redundancy: 6.5 % / Biso Wilson estimate: 20.35 Å2 / CC1/2: 1 / Net I/σ(I): 13.2 |
| Reflection shell | Resolution: 1.54→1.57 Å / Mean I/σ(I) obs: 1.1 / Num. unique obs: 3162 / CC1/2: 0.6 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 6SOV Resolution: 1.54→63.87 Å / SU ML: 0.1928 / Cross valid method: FREE R-VALUE / σ(F): 0.06 / Phase error: 24.6142
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 27 Å2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.54→63.87 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
|
 Movie
Movie Controller
Controller
















 PDBj
PDBj

















