+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 6y1z | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Mouse serotonin 5HT3 receptor in complex with palonosetron | ||||||||||||
 Components Components | 5-hydroxytryptamine receptor 3A | ||||||||||||
 Keywords Keywords | TRANSPORT PROTEIN / Receptor / Pentameric ligand-gated ion channel / antiemetic | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationNeurotransmitter receptors and postsynaptic signal transmission / serotonin-gated cation-selective signaling pathway / serotonin-activated cation-selective channel complex / serotonin-gated monoatomic cation channel activity / serotonin receptor signaling pathway / excitatory extracellular ligand-gated monoatomic ion channel activity / transmembrane transporter complex / serotonin binding / : / cleavage furrow ...Neurotransmitter receptors and postsynaptic signal transmission / serotonin-gated cation-selective signaling pathway / serotonin-activated cation-selective channel complex / serotonin-gated monoatomic cation channel activity / serotonin receptor signaling pathway / excitatory extracellular ligand-gated monoatomic ion channel activity / transmembrane transporter complex / serotonin binding / : / cleavage furrow / ligand-gated monoatomic ion channel activity involved in regulation of presynaptic membrane potential / regulation of membrane potential / transmitter-gated monoatomic ion channel activity involved in regulation of postsynaptic membrane potential / presynaptic membrane / monoatomic ion transmembrane transport / chemical synaptic transmission / postsynaptic membrane / neuron projection / axon / neuronal cell body / synapse / glutamatergic synapse / identical protein binding / plasma membrane Similarity search - Function | ||||||||||||
| Biological species |  | ||||||||||||
| Method | ELECTRON MICROSCOPY / single particle reconstruction / cryo EM / Resolution: 2.82 Å | ||||||||||||
 Authors Authors | Zarkadas, E. / Perot, J. / Nury, H. | ||||||||||||
| Funding support |  France, 1items France, 1items
| ||||||||||||
 Citation Citation |  Journal: Structure / Year: 2020 Journal: Structure / Year: 2020Title: The Binding of Palonosetron and Other Antiemetic Drugs to the Serotonin 5-HT3 Receptor. Authors: Eleftherios Zarkadas / Hong Zhang / Wensheng Cai / Gregory Effantin / Jonathan Perot / Jacques Neyton / Christophe Chipot / Guy Schoehn / Francois Dehez / Hugues Nury /    Abstract: Inaccurately perceived as niche drugs, antiemetics are key elements of cancer treatment alleviating the most dreaded side effect of chemotherapy. Serotonin 5-HT3 receptor antagonists are the most ...Inaccurately perceived as niche drugs, antiemetics are key elements of cancer treatment alleviating the most dreaded side effect of chemotherapy. Serotonin 5-HT3 receptor antagonists are the most commonly prescribed class of drugs to control chemotherapy-induced nausea and vomiting. These antagonists have been clinically successful drugs since the 1980s, yet our understanding of how they operate at the molecular level has been hampered by the difficulty of obtaining structures of drug-receptor complexes. Here, we report the cryoelectron microscopy structure of the palonosetron-bound 5-HT3 receptor. We investigate the binding of palonosetron, granisetron, dolasetron, ondansetron, and cilansetron using molecular dynamics, covering the whole set of antagonists used in clinical practice. The structural and computational results yield detailed atomic insight into the binding modes of the drugs. In light of our data, we establish a comprehensive framework underlying the inhibition mechanism by the -setron drug family. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  6y1z.cif.gz 6y1z.cif.gz | 379.3 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb6y1z.ent.gz pdb6y1z.ent.gz | 306.7 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  6y1z.json.gz 6y1z.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/y1/6y1z https://data.pdbj.org/pub/pdb/validation_reports/y1/6y1z ftp://data.pdbj.org/pub/pdb/validation_reports/y1/6y1z ftp://data.pdbj.org/pub/pdb/validation_reports/y1/6y1z | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  10673MC M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
-Protein , 1 types, 5 molecules ADBCE
| #1: Protein | Mass: 63682.719 Da / Num. of mol.: 5 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Homo sapiens (human) / References: UniProt: P23979 Homo sapiens (human) / References: UniProt: P23979 |
|---|
-Sugars , 3 types, 15 molecules 
| #2: Polysaccharide | beta-D-mannopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-2-acetamido-2-deoxy-beta- ...beta-D-mannopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose Source method: isolated from a genetically manipulated source #3: Polysaccharide | 2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose Source method: isolated from a genetically manipulated source #6: Sugar | ChemComp-NAG / |
|---|
-Non-polymers , 3 types, 15 molecules 
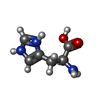
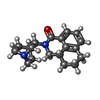


| #4: Chemical | ChemComp-TRP / #5: Chemical | ChemComp-HIS / #7: Chemical | ChemComp-O7B / ( |
|---|
-Details
| Has ligand of interest | Y |
|---|---|
| Has protein modification | Y |
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method: single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: Mouse serotonin 5-HT3 receptor in complex with palonosetron Type: COMPLEX / Entity ID: #1 / Source: RECOMBINANT |
|---|---|
| Source (natural) | Organism:  |
| Source (recombinant) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Buffer solution | pH: 7.4 |
| Specimen | Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: YES |
| Vitrification | Instrument: FEI VITROBOT MARK IV / Cryogen name: ETHANE / Humidity: 100 % |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TITAN KRIOS |
| Electron gun | Electron source:  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD |
| Image recording | Electron dose: 53 e/Å2 / Film or detector model: GATAN K2 SUMMIT (4k x 4k) |
- Processing
Processing
| Software | Name: PHENIX / Version: 1.17.1_3660: / Classification: refinement | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EM software |
| ||||||||||||||||||||||||
| CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION | ||||||||||||||||||||||||
| Symmetry | Point symmetry: C5 (5 fold cyclic) | ||||||||||||||||||||||||
| 3D reconstruction | Resolution: 2.82 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 127971 / Symmetry type: POINT | ||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller













 PDBj
PDBj






