[English] 日本語
 Yorodumi
Yorodumi- PDB-6xr4: Integrative in situ structure of Parkinsons disease-linked human LRRK2 -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 6xr4 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Integrative in situ structure of Parkinsons disease-linked human LRRK2 | |||||||||
 Components Components | Leucine-rich repeat serine/threonine-protein kinase 2 | |||||||||
 Keywords Keywords | SIGNALING PROTEIN / TRANSFERASE / kinase / GTPase / Parkinson's Disease / pseudo-kinase | |||||||||
| Function / homology |  Function and homology information Function and homology informationcaveola neck / : / beta-catenin destruction complex binding / regulation of branching morphogenesis of a nerve / Wnt signalosome assembly / regulation of kidney size / regulation of cell projection organization / tangential migration from the subventricular zone to the olfactory bulb / GTP-dependent protein kinase activity / regulation of SNARE complex assembly ...caveola neck / : / beta-catenin destruction complex binding / regulation of branching morphogenesis of a nerve / Wnt signalosome assembly / regulation of kidney size / regulation of cell projection organization / tangential migration from the subventricular zone to the olfactory bulb / GTP-dependent protein kinase activity / regulation of SNARE complex assembly / regulation of neuroblast proliferation / regulation of ER to Golgi vesicle-mediated transport / protein localization to endoplasmic reticulum exit site / peroxidase inhibitor activity / negative regulation of late endosome to lysosome transport / regulation of mitochondrial depolarization / : / positive regulation of dopamine receptor signaling pathway / amphisome / regulation of synaptic vesicle transport / regulation of lysosomal lumen pH / regulation of CAMKK-AMPK signaling cascade / co-receptor binding / negative regulation of GTPase activity / regulation of dopamine receptor signaling pathway / positive regulation of microglial cell activation / regulation of neuron maturation / regulation of retrograde transport, endosome to Golgi / positive regulation of synaptic vesicle endocytosis / negative regulation of excitatory postsynaptic potential / cytoplasmic side of mitochondrial outer membrane / negative regulation of autophagosome assembly / olfactory bulb development / JUN kinase kinase kinase activity / neuron projection arborization / striatum development / multivesicular body, internal vesicle / regulation of dendritic spine morphogenesis / mitochondrion localization / protein localization to mitochondrion / cellular response to dopamine / positive regulation of mitochondrial outer membrane permeabilization involved in apoptotic signaling pathway / endoplasmic reticulum organization / positive regulation of protein autoubiquitination / Wnt signalosome / negative regulation of protein processing / positive regulation of programmed cell death / GTP metabolic process / regulation of canonical Wnt signaling pathway / syntaxin-1 binding / regulation of reactive oxygen species metabolic process / lysosome organization / Golgi-associated vesicle / clathrin binding / PTK6 promotes HIF1A stabilization / negative regulation of macroautophagy / regulation of cAMP/PKA signal transduction / regulation of mitochondrial fission / protein kinase A binding / neuromuscular junction development / regulation of locomotion / regulation of synaptic vesicle exocytosis / Golgi organization / intracellular distribution of mitochondria / microvillus / exploration behavior / endoplasmic reticulum exit site / autolysosome / locomotory exploration behavior / negative regulation of Notch signaling pathway / MAP kinase kinase kinase activity / regulation of synaptic vesicle endocytosis / canonical Wnt signaling pathway / regulation of synaptic transmission, glutamatergic / negative regulation of endoplasmic reticulum stress-induced intrinsic apoptotic signaling pathway / Rho protein signal transduction / presynaptic cytosol / neuron projection morphogenesis / phagocytic vesicle / cellular response to manganese ion / JNK cascade / positive regulation of autophagy / dendrite cytoplasm / tubulin binding / GTPase activator activity / cellular response to starvation / positive regulation of protein ubiquitination / SNARE binding / determination of adult lifespan / regulation of membrane potential / cellular response to reactive oxygen species / mitochondrion organization / excitatory postsynaptic potential / trans-Golgi network / calcium-mediated signaling / regulation of protein stability / autophagy / small GTPase binding / mitochondrial membrane / regulation of autophagy Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | ELECTRON MICROSCOPY / subtomogram averaging / cryo EM / Resolution: 14 Å | |||||||||
 Authors Authors | Villa, E. / Lasker, K. / Audagnotto, M. | |||||||||
| Funding support |  United States, 2items United States, 2items
| |||||||||
 Citation Citation |  Journal: Cell / Year: 2020 Journal: Cell / Year: 2020Title: The In Situ Structure of Parkinson's Disease-Linked LRRK2. Authors: Reika Watanabe / Robert Buschauer / Jan Böhning / Martina Audagnotto / Keren Lasker / Tsan-Wen Lu / Daniela Boassa / Susan Taylor / Elizabeth Villa /  Abstract: Mutations in leucine-rich repeat kinase 2 (LRRK2) are the most frequent cause of familial Parkinson's disease. LRRK2 is a multi-domain protein containing a kinase and GTPase. Using correlative light ...Mutations in leucine-rich repeat kinase 2 (LRRK2) are the most frequent cause of familial Parkinson's disease. LRRK2 is a multi-domain protein containing a kinase and GTPase. Using correlative light and electron microscopy, in situ cryo-electron tomography, and subtomogram analysis, we reveal a 14-Å structure of LRRK2 bearing a pathogenic mutation that oligomerizes as a right-handed double helix around microtubules, which are left-handed. Using integrative modeling, we determine the architecture of LRRK2, showing that the GTPase and kinase are in close proximity, with the GTPase closer to the microtubule surface, whereas the kinase is exposed to the cytoplasm. We identify two oligomerization interfaces mediated by non-catalytic domains. Mutation of one of these abolishes LRRK2 microtubule-association. Our work demonstrates the power of cryo-electron tomography to generate models of previously unsolved structures in their cellular environment. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  6xr4.cif.gz 6xr4.cif.gz | 3.6 MB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb6xr4.ent.gz pdb6xr4.ent.gz | 2.4 MB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  6xr4.json.gz 6xr4.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/xr/6xr4 https://data.pdbj.org/pub/pdb/validation_reports/xr/6xr4 ftp://data.pdbj.org/pub/pdb/validation_reports/xr/6xr4 ftp://data.pdbj.org/pub/pdb/validation_reports/xr/6xr4 | HTTPS FTP |
|---|
-Related structure data
| Related structure data | 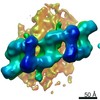 20825MC M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
| Number of models | 53 |
- Components
Components
| #1: Protein | Mass: 286450.719 Da / Num. of mol.: 2 / Mutation: I2020T / Source method: isolated from a natural source / Source: (natural)  Homo sapiens (human) / Cell line: HEK293 Homo sapiens (human) / Cell line: HEK293References: UniProt: Q5S007, non-specific serine/threonine protein kinase, Hydrolases; Acting on acid anhydrides; Acting on GTP to facilitate cellular and subcellular movement |
|---|
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: CELL / 3D reconstruction method: subtomogram averaging |
- Sample preparation
Sample preparation
| Component | Name: HEK293 cell / Type: CELL Details: Dimer within the oligomer of microtubule-bound human LRRK2-I2020T in HEK cells Entity ID: all / Source: NATURAL |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) / Cell: HEK293 / Cellular location: cytosol Homo sapiens (human) / Cell: HEK293 / Cellular location: cytosol |
| Buffer solution | pH: 7 |
| Specimen | Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: YES Details: FIB-milled cellular samples expressing Parkinson's disease-related mutant LRRK2 (I2020T) proteins in human embryonic kidney cells (HEK293 cells) |
| Specimen support | Grid material: GOLD / Grid mesh size: 200 divisions/in. / Grid type: Quantifoil |
| Vitrification | Cryogen name: ETHANE-PROPANE |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Tecnai Polara / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI POLARA 300 |
| Electron gun | Electron source:  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD |
| Image recording | Electron dose: 2 e/Å2 / Detector mode: COUNTING / Film or detector model: GATAN K2 SUMMIT (4k x 4k) |
| EM imaging optics | Energyfilter name: GIF Quantum SE / Energyfilter slit width: 20 eV |
- Processing
Processing
| EM software |
| |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTF correction | Type: PHASE FLIPPING ONLY | |||||||||||||||
| Symmetry | Point symmetry: C1 (asymmetric) | |||||||||||||||
| 3D reconstruction | Resolution: 14 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 4307 Details: Each model in the conformation ensemble represents a cluster. Models are arranged sequentially from lowest to highest cluster RMSD. Model 1 is the best representative model. Model-Cluster ...Details: Each model in the conformation ensemble represents a cluster. Models are arranged sequentially from lowest to highest cluster RMSD. Model 1 is the best representative model. Model-Cluster correpondence is as follows: Model 1 = Cluster 20 Model 2 = Cluster 16 Model 3 = Cluster 24 Model 4 = Cluster 32 Model 5 = Cluster 14 Model 6 = Cluster 1 Model 7 = Cluster 44 Model 8 = Cluster 11 Model 9 = Cluster 3 Model 10 = Cluster 22 Model 11 = Cluster 4 Model 12 = Cluster 12 Model 13 = Cluster 19 Model 14 = Cluster 7 Model 15 = Cluster 31 Model 16 = Cluster 26 Model 17 = Cluster 52 Model 18 = Cluster 2 Model 19 = Cluster 45 Model 20 = Cluster 8 Model 21 = Cluster 5 Model 22 = Cluster 23 Model 23 = Cluster 42 Model 24 = Cluster 25 Model 25 = Cluster 43 Model 26 = Cluster 27 Model 27 = Cluster 39 Model 28 = Cluster 6 Model 29 = Cluster 13 Model 30 = Cluster 28 Model 31 = Cluster 21 Model 32 = Cluster 10 Model 33 = Cluster 53 Model 34 = Cluster 30 Model 35 = Cluster 35 Model 36 = Cluster 38 Model 37 = Cluster 33 Model 38 = Cluster 40 Model 39 = Cluster 37 Model 40 = Cluster 29 Model 41 = Cluster 51 Model 42 = Cluster 41 Model 43 = Cluster 15 Model 44 = Cluster 9 Model 45 = Cluster 17 Model 46 = Cluster 50 Model 47 = Cluster 48 Model 48 = Cluster 49 Model 49 = Cluster 18 Model 50 = Cluster 46 Model 51 = Cluster 36 Model 52 = Cluster 47 Model 53 = Cluster 34 Symmetry type: POINT | |||||||||||||||
| EM volume selection | Num. of tomograms: 12 / Num. of volumes extracted: 11508 | |||||||||||||||
| Atomic model building | Protocol: OTHER | |||||||||||||||
| Refinement | Details: Each model in the conformation ensemble represents a cluster. Models are arranged sequentially from lowest to highest cluster RMSD. Model 1 is the best representative model. Model-Cluster ...Details: Each model in the conformation ensemble represents a cluster. Models are arranged sequentially from lowest to highest cluster RMSD. Model 1 is the best representative model. Model-Cluster correpondence is as follows: Model 1 = Cluster 20 Model 2 = Cluster 16 Model 3 = Cluster 24 Model 4 = Cluster 32 Model 5 = Cluster 14 Model 6 = Cluster 1 Model 7 = Cluster 44 Model 8 = Cluster 11 Model 9 = Cluster 3 Model 10 = Cluster 22 Model 11 = Cluster 4 Model 12 = Cluster 12 Model 13 = Cluster 19 Model 14 = Cluster 7 Model 15 = Cluster 31 Model 16 = Cluster 26 Model 17 = Cluster 52 Model 18 = Cluster 2 Model 19 = Cluster 45 Model 20 = Cluster 8 Model 21 = Cluster 5 Model 22 = Cluster 23 Model 23 = Cluster 42 Model 24 = Cluster 25 Model 25 = Cluster 43 Model 26 = Cluster 27 Model 27 = Cluster 39 Model 28 = Cluster 6 Model 29 = Cluster 13 Model 30 = Cluster 28 Model 31 = Cluster 21 Model 32 = Cluster 10 Model 33 = Cluster 53 Model 34 = Cluster 30 Model 35 = Cluster 35 Model 36 = Cluster 38 Model 37 = Cluster 33 Model 38 = Cluster 40 Model 39 = Cluster 37 Model 40 = Cluster 29 Model 41 = Cluster 51 Model 42 = Cluster 41 Model 43 = Cluster 15 Model 44 = Cluster 9 Model 45 = Cluster 17 Model 46 = Cluster 50 Model 47 = Cluster 48 Model 48 = Cluster 49 Model 49 = Cluster 18 Model 50 = Cluster 46 Model 51 = Cluster 36 Model 52 = Cluster 47 Model 53 = Cluster 34 |
 Movie
Movie Controller
Controller













 PDBj
PDBj







