+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 6t72 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
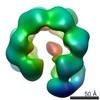
| Title | Structure of the RsaA N-terminal domain bound to LPS | |||||||||
 Components Components | S-layer protein | |||||||||
 Keywords Keywords | STRUCTURAL PROTEIN / S-layer LPS RsaA | |||||||||
| Function / homology | RsaA N-terminal domain / S-layer / RTX calcium-binding nonapeptide repeat / RTX calcium-binding nonapeptide repeat (4 copies) / Serralysin-like metalloprotease, C-terminal / calcium ion binding / extracellular region / S-layer protein Function and homology information Function and homology information | |||||||||
| Biological species |  Caulobacter vibrioides CB15 (bacteria) Caulobacter vibrioides CB15 (bacteria) | |||||||||
| Method | ELECTRON MICROSCOPY / single particle reconstruction / cryo EM / Resolution: 3.7 Å | |||||||||
 Authors Authors | von Kuegelgen, A. / Bharat, T.A.M. | |||||||||
| Funding support |  United Kingdom, 1items United Kingdom, 1items
| |||||||||
 Citation Citation |  Journal: Cell / Year: 2020 Journal: Cell / Year: 2020Title: In Situ Structure of an Intact Lipopolysaccharide-Bound Bacterial Surface Layer. Authors: Andriko von Kügelgen / Haiping Tang / Gail G Hardy / Danguole Kureisaite-Ciziene / Yves V Brun / Phillip J Stansfeld / Carol V Robinson / Tanmay A M Bharat /    Abstract: Most bacterial and all archaeal cells are encapsulated by a paracrystalline, protective, and cell-shape-determining proteinaceous surface layer (S-layer). On Gram-negative bacteria, S-layers are ...Most bacterial and all archaeal cells are encapsulated by a paracrystalline, protective, and cell-shape-determining proteinaceous surface layer (S-layer). On Gram-negative bacteria, S-layers are anchored to cells via lipopolysaccharide. Here, we report an electron cryomicroscopy structure of the Caulobacter crescentus S-layer bound to the O-antigen of lipopolysaccharide. Using native mass spectrometry and molecular dynamics simulations, we deduce the length of the O-antigen on cells and show how lipopolysaccharide binding and S-layer assembly is regulated by calcium. Finally, we present a near-atomic resolution in situ structure of the complete S-layer using cellular electron cryotomography, showing S-layer arrangement at the tip of the O-antigen. A complete atomic structure of the S-layer shows the power of cellular tomography for in situ structural biology and sheds light on a very abundant class of self-assembling molecules with important roles in prokaryotic physiology with marked potential for synthetic biology and surface-display applications. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  6t72.cif.gz 6t72.cif.gz | 63.2 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb6t72.ent.gz pdb6t72.ent.gz | 44.7 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  6t72.json.gz 6t72.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  6t72_validation.pdf.gz 6t72_validation.pdf.gz | 1.1 MB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  6t72_full_validation.pdf.gz 6t72_full_validation.pdf.gz | 1.1 MB | Display | |
| Data in XML |  6t72_validation.xml.gz 6t72_validation.xml.gz | 15.1 KB | Display | |
| Data in CIF |  6t72_validation.cif.gz 6t72_validation.cif.gz | 20.4 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/t7/6t72 https://data.pdbj.org/pub/pdb/validation_reports/t7/6t72 ftp://data.pdbj.org/pub/pdb/validation_reports/t7/6t72 ftp://data.pdbj.org/pub/pdb/validation_reports/t7/6t72 | HTTPS FTP |
-Related structure data
| Related structure data |  10389MC  6z7pC M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 | x 14
|
- Components
Components
| #1: Protein | Mass: 25820.354 Da / Num. of mol.: 1 / Mutation: TEV site added at the position 250 Source method: isolated from a genetically manipulated source Details: LPS O-antigen bound to protein / Source: (gene. exp.)  Caulobacter vibrioides CB15 (bacteria) / Gene: rsaA, CC_1007 / Production host: Caulobacter vibrioides CB15 (bacteria) / Gene: rsaA, CC_1007 / Production host:  Caulobacter vibrioides CB15 (bacteria) / References: UniProt: P35828 Caulobacter vibrioides CB15 (bacteria) / References: UniProt: P35828 | ||
|---|---|---|---|
| #2: Polysaccharide | 4-acetamido-4,6-dideoxy-alpha-D-mannopyranose-(1-3)-4-acetamido-4,6-dideoxy-alpha-D-mannopyranose- ...4-acetamido-4,6-dideoxy-alpha-D-mannopyranose-(1-3)-4-acetamido-4,6-dideoxy-alpha-D-mannopyranose-(1-3)-beta-D-mannopyranose-(1-3)-4-acetamido-4,6-dideoxy-alpha-D-mannopyranose-(1-3)-4-acetamido-4,6-dideoxy-alpha-D-mannopyranose-(1-3)-beta-D-mannopyranose-(1-3)-4-acetamido-4,6-dideoxy-alpha-D-mannopyranose-(1-3)-4-acetamido-4,6-dideoxy-alpha-D-mannopyranose-(1-3)-beta-D-mannopyranose-(1-3)-4-acetamido-4,6-dideoxy-alpha-D-mannopyranose-(1-3)-4-acetamido-4,6-dideoxy-alpha-D-mannopyranose-(1-3)-beta-D-mannopyranose | ||
| #3: Chemical | | Has ligand of interest | Y | |
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method: single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: Structure of RsaA N-terminal domain bound to LPS / Type: COMPLEX / Details: Structure of RsaA N-terminal domain bound to LPS / Entity ID: #1 / Source: NATURAL | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular weight | Value: 0.577 MDa / Experimental value: YES | |||||||||||||||||||||||||
| Source (natural) | Organism:  Caulobacter vibrioides CB15 (bacteria) / Strain: YB1001 Caulobacter vibrioides CB15 (bacteria) / Strain: YB1001 | |||||||||||||||||||||||||
| Buffer solution | pH: 7.5 Details: Buffer solutions were prepared fresh from sterile filtered concentrated stocksolutions. Solutions were filtered through a 0.22 um filter to avoid microbial contamination and degassed using a ...Details: Buffer solutions were prepared fresh from sterile filtered concentrated stocksolutions. Solutions were filtered through a 0.22 um filter to avoid microbial contamination and degassed using a vacuum fold pump. The pH of the HEPES stock solution was adjusted with sodium hydroxide at 4 deg C. | |||||||||||||||||||||||||
| Buffer component |
| |||||||||||||||||||||||||
| Specimen | Conc.: 2.5 mg/ml / Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: YES / Details: RsaA N-terminal domain with LPS | |||||||||||||||||||||||||
| Specimen support | Details: 20 seconds, 15 mA / Grid material: COPPER/RHODIUM / Grid mesh size: 200 divisions/in. / Grid type: Quantifoil R2/2 | |||||||||||||||||||||||||
| Vitrification | Instrument: FEI VITROBOT MARK IV / Cryogen name: ETHANE / Humidity: 100 % / Chamber temperature: 283.15 K Details: Vitrobot options: Blot time 3 seconds, Blot force -13,1, Wait time 10 seconds, Drain time 0.5 seconds, |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TITAN KRIOS / Details: EPU software |
| Electron gun | Electron source:  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD / Nominal magnification: 130000 X / Calibrated magnification: 130000 X / Nominal defocus max: -4000 nm / Nominal defocus min: -1000 nm / Calibrated defocus min: -1000 nm / Calibrated defocus max: -4000 nm / Cs: 2.7 mm / C2 aperture diameter: 50 µm / Alignment procedure: ZEMLIN TABLEAU |
| Specimen holder | Cryogen: NITROGEN / Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Temperature (max): 70 K / Temperature (min): 70 K |
| Image recording | Average exposure time: 8 sec. / Electron dose: 43 e/Å2 / Detector mode: COUNTING / Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Num. of grids imaged: 1 / Num. of real images: 2422 Details: Images were collected in movie-mode and subjected to 8 seconds of exposure where a total dose of 43 e-/A2 was applied, and 20 frames were recorded per movie. |
| EM imaging optics | Energyfilter name: GIF Quantum LS / Energyfilter slit width: 20 eV |
| Image scans | Width: 3838 / Height: 3710 / Movie frames/image: 20 / Used frames/image: 1-20 |
- Processing
Processing
| EM software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Image processing | Details: Movies were motion corrected and dose weighted with MotionCor2 (Zheng et al., 2017) implemented in Relion 3.0 (Zivanov et al., 2018). Contrast transfer functions (CTFs) of the resulting ...Details: Movies were motion corrected and dose weighted with MotionCor2 (Zheng et al., 2017) implemented in Relion 3.0 (Zivanov et al., 2018). Contrast transfer functions (CTFs) of the resulting motion corrected micrographs were estimated using CTFFIND4 (Rohou and Grigorieff, 2015). | ||||||||||||||||||||||||||||||||||||||||||||||||||
| CTF correction | Details: RELION refinement with in-built CTF correction. The function is similar to a Wiener filter, so amplitude correction included. Type: PHASE FLIPPING AND AMPLITUDE CORRECTION | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Particle selection | Num. of particles selected: 129633 Details: Particles were automatically picked from the motion and CTF corrected micrographs using the AutoPick function in Relion 3.0 (Zivanov et al., 2018). As particle reference a 3 dimensional ...Details: Particles were automatically picked from the motion and CTF corrected micrographs using the AutoPick function in Relion 3.0 (Zivanov et al., 2018). As particle reference a 3 dimensional reconstruction from an earlier dataset with different pixelsize was used which was reconstructed using an unbiased subtomogram average structure of the same sample. | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Point symmetry: C1 (asymmetric) | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 3D reconstruction | Resolution: 3.7 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 115776 / Algorithm: FOURIER SPACE Details: Particles from two main 3D classes containing 21 or 20 RsaA subunits were combined for a focused 3D auto refinement on the central 14 subunits using the output from the 3D classification as ...Details: Particles from two main 3D classes containing 21 or 20 RsaA subunits were combined for a focused 3D auto refinement on the central 14 subunits using the output from the 3D classification as a starting model. The final map was obtained from 115,776 particles and post-processed using a soft mask focused on the inner fourteen subunits. Num. of class averages: 2 / Symmetry type: POINT | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic model building | B value: 85.819 / Protocol: BACKBONE TRACE / Space: RECIPROCAL / Target criteria: Best fit Details: The carbon backbone of the RsaA protein was manually traced through a single subunit of the cryo-EM density using Coot (Emsley et al., 2010). Initially, side chains were assigned in regions ...Details: The carbon backbone of the RsaA protein was manually traced through a single subunit of the cryo-EM density using Coot (Emsley et al., 2010). Initially, side chains were assigned in regions with density corresponding to characteristic aromatic residues allowing us to deduce the register of the amino acid sequence in the map. Side chains for residues 2-243 of RsaA were thus assigned unambiguously and the structure was refined and manually rebuilt using Refmac5 (Murshudov et al., 2011) inside the CCP-EM (Burnley et al., 2017) software suite and Coot. |
 Movie
Movie Controller
Controller







 PDBj
PDBj

