[English] 日本語
 Yorodumi
Yorodumi- PDB-6slf: Nalpha-acylglutamine aminoacylase from Corynebacterium sp.releasi... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 6slf | ||||||
|---|---|---|---|---|---|---|---|
| Title | Nalpha-acylglutamine aminoacylase from Corynebacterium sp.releasing human axilla odorants co-crystallised with high affinity inhibitor | ||||||
 Components Components | N-alpha-acyl-glutamine aminoacylase | ||||||
 Keywords Keywords | HYDROLASE / aminoacylase / inhibitor / complex / tetramer | ||||||
| Function / homology |  Function and homology information Function and homology information | ||||||
| Biological species |  Corynebacterium striatum (bacteria) Corynebacterium striatum (bacteria) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / MOLECULAR REPLACEMENT /  molecular replacement / Resolution: 1.75 Å molecular replacement / Resolution: 1.75 Å | ||||||
 Authors Authors | Natsch, A. / Emter, R. | ||||||
 Citation Citation |  Journal: Philos.Trans.R.Soc.Lond.B Biol.Sci. / Year: 2020 Journal: Philos.Trans.R.Soc.Lond.B Biol.Sci. / Year: 2020Title: The specific biochemistry of human axilla odour formation viewed in an evolutionary context. Authors: Natsch, A. / Emter, R. #1: Journal: J.Biol.Chem. / Year: 2003 Title: A specific bacterial aminoacylase cleaves odorant precursors secreted in the human axilla. Authors: Natsch, A. / Gfeller, H. / Gygax, P. / Schmid, J. / Acuna, G. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  6slf.cif.gz 6slf.cif.gz | 335.4 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb6slf.ent.gz pdb6slf.ent.gz | 268.5 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  6slf.json.gz 6slf.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/sl/6slf https://data.pdbj.org/pub/pdb/validation_reports/sl/6slf ftp://data.pdbj.org/pub/pdb/validation_reports/sl/6slf ftp://data.pdbj.org/pub/pdb/validation_reports/sl/6slf | HTTPS FTP |
|---|
-Related structure data
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
-Protein , 1 types, 4 molecules ABCD
| #1: Protein | Mass: 43534.402 Da / Num. of mol.: 4 Source method: isolated from a genetically manipulated source Details: Isolate from skin in human axillae / Source: (gene. exp.)  Corynebacterium striatum (bacteria) / Gene: agaA / Production host: Corynebacterium striatum (bacteria) / Gene: agaA / Production host:  |
|---|
-Non-polymers , 6 types, 1040 molecules 
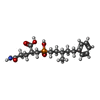









| #2: Chemical | ChemComp-ZN / #3: Chemical | ChemComp-LJ8 / ( #4: Chemical | #5: Chemical | ChemComp-ACT / #6: Chemical | ChemComp-BTB / | #7: Water | ChemComp-HOH / | |
|---|
-Details
| Has ligand of interest | Y |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.56 Å3/Da / Density % sol: 51.92 % |
|---|---|
| Crystal grow | Temperature: 296 K / Method: vapor diffusion, sitting drop / pH: 6.5 Details: 90microL of 30% PEG4K, 0.22M sodium acetate, 0.1M Tris pH 8.5 + 10microL of Index screen (Hampton Research) containing 0.2M ammonium sulphate, 0.1M Bis-Tris pH6.5, 25%PEG3350 |
-Data collection
| Diffraction | Mean temperature: 296 K / Ambient temp details: not exactly reported / Serial crystal experiment: N |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  ESRF ESRF  / Beamline: ID23-1 / Wavelength: 1.282 Å / Beamline: ID23-1 / Wavelength: 1.282 Å |
| Detector | Type: ADSC QUANTUM 1 / Detector: CCD / Date: Sep 1, 2006 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1.282 Å / Relative weight: 1 |
| Reflection | Resolution: 1.5→29.58 Å / Num. obs: 164787 / % possible obs: 100 % / Redundancy: 6.93 % / Biso Wilson estimate: 33.5 Å2 / Rmerge(I) obs: 0.0108 / Net I/σ(I): 8.3 |
| Reflection shell | Resolution: 1.5→1.8 Å / Rmerge(I) obs: 0.0418 / Num. unique obs: 164787 / % possible all: 99.9 |
-Phasing
| Phasing | Method:  molecular replacement molecular replacement |
|---|
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 1XMB, 1YSJ Resolution: 1.75→29.58 Å / Cor.coef. Fo:Fc: 0.964 / Cor.coef. Fo:Fc free: 0.955 / SU B: 2.249 / SU ML: 0.073 / Cross valid method: THROUGHOUT / σ(F): 0 / ESU R: 0.112 / ESU R Free: 0.106 / Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.4 Å | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso max: 64.64 Å2 / Biso mean: 26.211 Å2 / Biso min: 10.79 Å2
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: final / Resolution: 1.75→29.58 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 1.75→1.795 Å / Rfactor Rfree error: 0 / Total num. of bins used: 20
|
 Movie
Movie Controller
Controller










 PDBj
PDBj





