[English] 日本語
 Yorodumi
Yorodumi- PDB-6pqp: Cryo-EM structure of the human TRPA1 ion channel in complex with ... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 6pqp | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of the human TRPA1 ion channel in complex with the covalent agonist BITC | |||||||||
 Components Components | Transient receptor potential cation channel subfamily A member 1 | |||||||||
 Keywords Keywords | TRANSPORT PROTEIN / Ion channel / TRP channel / TRPA channel / TRPA1 channel / irritant sensing / BITC / membrane protein | |||||||||
| Function / homology |  Function and homology information Function and homology informationregulation of blood circulation / positive regulation of monoatomic anion transport / cellular response to food / temperature-gated cation channel activity / regulation of neuronal action potential / cellular response to carbon dioxide / osmolarity-sensing monoatomic cation channel activity / stereocilium bundle / detection of chemical stimulus involved in sensory perception of pain / urinary bladder smooth muscle contraction ...regulation of blood circulation / positive regulation of monoatomic anion transport / cellular response to food / temperature-gated cation channel activity / regulation of neuronal action potential / cellular response to carbon dioxide / osmolarity-sensing monoatomic cation channel activity / stereocilium bundle / detection of chemical stimulus involved in sensory perception of pain / urinary bladder smooth muscle contraction / thermoception / cellular response to toxic substance / cellular response to caffeine / response to pain / TRP channels / calcium ion transmembrane import into cytosol / cellular response to cold / intracellularly gated calcium channel activity / detection of mechanical stimulus involved in sensory perception of pain / positive regulation of insulin secretion involved in cellular response to glucose stimulus / voltage-gated calcium channel activity / monoatomic ion transport / sensory perception of pain / response to cold / calcium ion transmembrane transport / calcium channel activity / cellular response to hydrogen peroxide / intracellular calcium ion homeostasis / cellular response to heat / channel activity / protein homotetramerization / cell surface receptor signaling pathway / apical plasma membrane / response to xenobiotic stimulus / axon / identical protein binding / plasma membrane Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | ELECTRON MICROSCOPY / single particle reconstruction / cryo EM / Resolution: 3.06 Å | |||||||||
 Authors Authors | Suo, Y. / Wang, Z. / Zubcevic, L. / Hsu, A.L. / He, Q. / Borgnia, M.J. / Ji, R.-R. / Lee, S.-Y. | |||||||||
| Funding support |  United States, 1items United States, 1items
| |||||||||
 Citation Citation |  Journal: Neuron / Year: 2020 Journal: Neuron / Year: 2020Title: Structural Insights into Electrophile Irritant Sensing by the Human TRPA1 Channel. Authors: Yang Suo / Zilong Wang / Lejla Zubcevic / Allen L Hsu / Qianru He / Mario J Borgnia / Ru-Rong Ji / Seok-Yong Lee /  Abstract: Transient receptor potential channel subfamily A member 1 (TRPA1) is a Ca-permeable cation channel that serves as one of the primary sensors of environmental irritants and noxious substances. Many ...Transient receptor potential channel subfamily A member 1 (TRPA1) is a Ca-permeable cation channel that serves as one of the primary sensors of environmental irritants and noxious substances. Many TRPA1 agonists are electrophiles that are recognized by TRPA1 via covalent bond modifications of specific cysteine residues located in the cytoplasmic domains. However, a mechanistic understanding of electrophile sensing by TRPA1 has been limited due to a lack of high-resolution structural information. Here, we present the cryoelectron microscopy (cryo-EM) structures of nanodisc-reconstituted ligand-free TRPA1 and TRPA1 in complex with the covalent agonists JT010 and BITC at 2.8, 2.9, and 3.1 Å, respectively. Our structural and functional studies provide the molecular basis for electrophile recognition by the extraordinarily reactive C621 in TRPA1 and mechanistic insights into electrophile-dependent conformational changes in TRPA1. This work also provides a platform for future drug development targeting TRPA1. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  6pqp.cif.gz 6pqp.cif.gz | 500.3 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb6pqp.ent.gz pdb6pqp.ent.gz | 384.8 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  6pqp.json.gz 6pqp.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/pq/6pqp https://data.pdbj.org/pub/pdb/validation_reports/pq/6pqp ftp://data.pdbj.org/pub/pdb/validation_reports/pq/6pqp ftp://data.pdbj.org/pub/pdb/validation_reports/pq/6pqp | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  20450MC  6pqoC  6pqqC M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
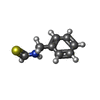
| #1: Protein | Mass: 131495.547 Da / Num. of mol.: 4 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: TRPA1, ANKTM1 / Production host: Homo sapiens (human) / Gene: TRPA1, ANKTM1 / Production host:  Homo sapiens (human) / References: UniProt: O75762 Homo sapiens (human) / References: UniProt: O75762#2: Polysaccharide | 2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose Source method: isolated from a genetically manipulated source #3: Chemical | ChemComp-9BE / #4: Chemical | ChemComp-6OU / [( #5: Chemical | ChemComp-LBN / Has ligand of interest | Y | Has protein modification | Y | |
|---|
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method: single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: Transient receptor potential cation channel subfamily A member 1 Type: COMPLEX / Entity ID: #1 / Source: RECOMBINANT |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Source (recombinant) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Buffer solution | pH: 8 |
| Specimen | Conc.: 0.5 mg/ml / Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: YES |
| Specimen support | Details: 15 mA / Grid material: GOLD / Grid mesh size: 300 divisions/in. / Grid type: UltrAuFoil |
| Vitrification | Instrument: FEI VITROBOT MARK II / Cryogen name: ETHANE / Humidity: 100 % / Chamber temperature: 277 K |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TITAN KRIOS |
| Electron gun | Electron source:  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD / Nominal magnification: 22500 X / Nominal defocus max: 2250 nm / Nominal defocus min: 750 nm / Cs: 2.7 mm / Alignment procedure: COMA FREE |
| Specimen holder | Cryogen: NITROGEN / Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER |
| Image recording | Average exposure time: 4.6 sec. / Electron dose: 60 e/Å2 / Detector mode: COUNTING / Film or detector model: GATAN K3 (6k x 4k) / Num. of grids imaged: 1 / Num. of real images: 4418 |
- Processing
Processing
| Software | Name: PHENIX / Version: 1.13_2998: / Classification: refinement | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EM software |
| ||||||||||||||||||||||||||||||||||||
| CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION | ||||||||||||||||||||||||||||||||||||
| Particle selection | Num. of particles selected: 396597 | ||||||||||||||||||||||||||||||||||||
| Symmetry | Point symmetry: C4 (4 fold cyclic) | ||||||||||||||||||||||||||||||||||||
| 3D reconstruction | Resolution: 3.06 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 74677 / Num. of class averages: 1 / Symmetry type: POINT | ||||||||||||||||||||||||||||||||||||
| Atomic model building | B value: 100 / Protocol: RIGID BODY FIT / Space: REAL | ||||||||||||||||||||||||||||||||||||
| Atomic model building | PDB-ID: 3J9P Accession code: 3J9P / Source name: PDB / Type: experimental model | ||||||||||||||||||||||||||||||||||||
| Refinement | Highest resolution: 3.06 Å |
 Movie
Movie Controller
Controller












 PDBj
PDBj