[English] 日本語
 Yorodumi
Yorodumi- PDB-6pow: Structure of human endotheial nitric oxide synthase heme domain i... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 6pow | ||||||
|---|---|---|---|---|---|---|---|
| Title | Structure of human endotheial nitric oxide synthase heme domain in complex with 7-(5-(Aminomethyl)pyridin-3-yl)-4-methylquinolin-2-amine | ||||||
 Components Components | Nitric oxide synthase, endothelial | ||||||
 Keywords Keywords | OXIDOREDUCTASE/INHIBITOR / nitric oxide synthase inhibitor / heme enzyme / OXIDOREDUCTASE / OXIDOREDUCTASE-INHIBITOR complex | ||||||
| Function / homology |  Function and homology information Function and homology informationregulation of the force of heart contraction by chemical signal / NOSIP mediated eNOS trafficking / negative regulation of muscle hyperplasia / NOSTRIN mediated eNOS trafficking / tetrahydrobiopterin metabolic process / smooth muscle hyperplasia / regulation of nervous system process / superoxide-generating NAD(P)H oxidase activity / ovulation from ovarian follicle / pulmonary valve morphogenesis ...regulation of the force of heart contraction by chemical signal / NOSIP mediated eNOS trafficking / negative regulation of muscle hyperplasia / NOSTRIN mediated eNOS trafficking / tetrahydrobiopterin metabolic process / smooth muscle hyperplasia / regulation of nervous system process / superoxide-generating NAD(P)H oxidase activity / ovulation from ovarian follicle / pulmonary valve morphogenesis / response to fluid shear stress / negative regulation of biomineral tissue development / Nitric oxide stimulates guanylate cyclase / regulation of systemic arterial blood pressure by endothelin / ROS and RNS production in phagocytes / tetrahydrobiopterin binding / arginine binding / aortic valve morphogenesis / endocardial cushion morphogenesis / ventricular septum morphogenesis / positive regulation of Notch signaling pathway / cadmium ion binding / negative regulation of calcium ion transport / negative regulation of potassium ion transport / negative regulation of platelet activation / actin monomer binding / positive regulation of blood vessel endothelial cell migration / nitric oxide mediated signal transduction / blood vessel remodeling / nitric-oxide synthase (NADPH) / nitric-oxide synthase activity / endothelial cell migration / L-arginine catabolic process / negative regulation of extrinsic apoptotic signaling pathway via death domain receptors / negative regulation of blood pressure / regulation of sodium ion transport / response to hormone / nitric oxide metabolic process / eNOS activation / Tetrahydrobiopterin (BH4) synthesis, recycling, salvage and regulation / nitric oxide biosynthetic process / homeostasis of number of cells within a tissue / removal of superoxide radicals / lung development / cell redox homeostasis / lipopolysaccharide-mediated signaling pathway / blood vessel diameter maintenance / VEGFR2 mediated vascular permeability / mitochondrion organization / establishment of localization in cell / potassium ion transport / negative regulation of smooth muscle cell proliferation / caveola / regulation of blood pressure / vasodilation / positive regulation of angiogenesis / endocytic vesicle membrane / calcium ion transport / FMN binding / NADP binding / flavin adenine dinucleotide binding / response to heat / angiogenesis / scaffold protein binding / High laminar flow shear stress activates signaling by PIEZO1 and PECAM1:CDH5:KDR in endothelial cells / response to lipopolysaccharide / in utero embryonic development / cytoskeleton / calmodulin binding / Extra-nuclear estrogen signaling / Golgi membrane / negative regulation of cell population proliferation / heme binding / positive regulation of gene expression / Golgi apparatus / nucleus / plasma membrane / cytoplasm / cytosol Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.15 Å MOLECULAR REPLACEMENT / Resolution: 2.15 Å | ||||||
 Authors Authors | Chreifi, G. / Li, H. / Poulos, T.L. | ||||||
| Funding support |  United States, 1items United States, 1items
| ||||||
 Citation Citation |  Journal: J.Med.Chem. / Year: 2020 Journal: J.Med.Chem. / Year: 2020Title: First Contact: 7-Phenyl-2-Aminoquinolines, Potent and Selective Neuronal Nitric Oxide Synthase Inhibitors That Target an Isoform-Specific Aspartate. Authors: Cinelli, M.A. / Reidl, C.T. / Li, H. / Chreifi, G. / Poulos, T.L. / Silverman, R.B. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  6pow.cif.gz 6pow.cif.gz | 674.4 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb6pow.ent.gz pdb6pow.ent.gz | 557.8 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  6pow.json.gz 6pow.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/po/6pow https://data.pdbj.org/pub/pdb/validation_reports/po/6pow ftp://data.pdbj.org/pub/pdb/validation_reports/po/6pow ftp://data.pdbj.org/pub/pdb/validation_reports/po/6pow | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  6pmvC  6pmwC  6pmxC  6pmyC  6pmzC  6pn0C  6pn1C  6pn2C  6pn3C  6pn4C  6pn5C  6pn6C  6pn7C  6pn8C  6pn9C  6pnaC  6pnbC  6pncC  6pndC  6pneC  6pnfC  6pngC  6pnhC  6po5C  6po7C  6po8C  6po9C  6poaC  6pobC  6pocC  6potC  6pouC  6povC  6poxC  6poyC  6pozC  6pp0C  6pp1C  6pp2C  6pp3C  6pp4C  4d1pS S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| 2 | 
| ||||||||
| Unit cell |
|
- Components
Components
-Protein , 1 types, 4 molecules ABCD
| #1: Protein | Mass: 49345.770 Da / Num. of mol.: 4 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Cell: endothelial / Gene: NOS3 / Plasmid: pCWori / Production host: Homo sapiens (human) / Cell: endothelial / Gene: NOS3 / Plasmid: pCWori / Production host:  |
|---|
-Non-polymers , 9 types, 657 molecules 
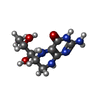
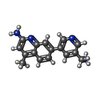














| #2: Chemical | ChemComp-HEM / #3: Chemical | #4: Chemical | ChemComp-OUA / #5: Chemical | ChemComp-BTB / #6: Chemical | #7: Chemical | #8: Chemical | ChemComp-CL / #9: Chemical | ChemComp-GD / #10: Water | ChemComp-HOH / | |
|---|
-Details
| Has ligand of interest | Y |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.69 Å3/Da / Density % sol: 54.3 % / Description: rods |
|---|---|
| Crystal grow | Temperature: 277 K / Method: vapor diffusion, sitting drop / pH: 7.5 Details: 10-12% PEG3350, 0.1M BIS-TRIS 0.2-0.3M MG ACETATE, 0.1M GdCl3 10% glycerol, 5 mM TCEP |
-Data collection
| Diffraction | Mean temperature: 100 K / Serial crystal experiment: N |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  ALS ALS  / Beamline: 5.0.2 / Wavelength: 1 Å / Beamline: 5.0.2 / Wavelength: 1 Å |
| Detector | Type: DECTRIS PILATUS 300K / Detector: PIXEL / Date: May 28, 2017 / Details: mirrors |
| Radiation | Monochromator: DOUBLE CRYSTAL SI(111) / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1 Å / Relative weight: 1 |
| Reflection | Resolution: 2.15→60 Å / Num. obs: 104668 / % possible obs: 99.2 % / Observed criterion σ(I): -3 / Redundancy: 4.1 % / CC1/2: 0.99 / Rmerge(I) obs: 0.148 / Rpim(I) all: 0.114 / Rsym value: 0.148 / Net I/σ(I): 6 |
| Reflection shell | Resolution: 2.15→2.21 Å / Redundancy: 4 % / Rmerge(I) obs: 1.328 / Mean I/σ(I) obs: 1.2 / Num. unique obs: 5115 / CC1/2: 0.53 / Rpim(I) all: 1.037 / Rsym value: 1.328 / % possible all: 98.2 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 4D1P Resolution: 2.15→59.694 Å / SU ML: 0.32 / Cross valid method: FREE R-VALUE / σ(F): 0 / Phase error: 32.47
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.15→59.694 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Refine-ID: X-RAY DIFFRACTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group |
|
 Movie
Movie Controller
Controller






















 PDBj
PDBj

















