+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 6pbs | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
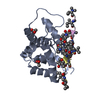
| Title | Structure of ClpC1-NTD in complex with Ecumicin | ||||||||||||
 Components Components |
| ||||||||||||
 Keywords Keywords | CHAPERONE/ANTIBIOTIC / ClpC1-NTD / Ecumicin / ATPase / Mycobacterium tuberculosis / CHAPERONE-ANTIBIOTIC complex | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationprotein folding chaperone / peptidoglycan-based cell wall / protein homodimerization activity / ATP hydrolysis activity / ATP binding / plasma membrane / cytosol Similarity search - Function | ||||||||||||
| Biological species |   Nonomuraea sp. MJM5123 (bacteria) Nonomuraea sp. MJM5123 (bacteria) | ||||||||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.5 Å MOLECULAR REPLACEMENT / Resolution: 2.5 Å | ||||||||||||
 Authors Authors | Abad-Zapatero, C. / Wolf, N.M. | ||||||||||||
 Citation Citation |  Journal: Acta Crystallogr D Struct Biol / Year: 2020 Journal: Acta Crystallogr D Struct Biol / Year: 2020Title: Structure of the N-terminal domain of ClpC1 in complex with the antituberculosis natural product ecumicin reveals unique binding interactions. Authors: Wolf, N.M. / Lee, H. / Zagal, D. / Nam, J.W. / Oh, D.C. / Lee, H. / Suh, J.W. / Pauli, G.F. / Cho, S. / Abad-Zapatero, C. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  6pbs.cif.gz 6pbs.cif.gz | 425.6 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb6pbs.ent.gz pdb6pbs.ent.gz | 359.5 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  6pbs.json.gz 6pbs.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/pb/6pbs https://data.pdbj.org/pub/pdb/validation_reports/pb/6pbs ftp://data.pdbj.org/pub/pdb/validation_reports/pb/6pbs ftp://data.pdbj.org/pub/pdb/validation_reports/pb/6pbs | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  6pbaSC  6pbqC  6ucrC S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
- Components
Components
| #1: Protein | Mass: 17529.131 Da / Num. of mol.: 12 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   #2: Protein/peptide | #3: Chemical | ChemComp-ACT / #4: Water | ChemComp-HOH / | Has ligand of interest | Y | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.36 Å3/Da / Density % sol: 47.82 % |
|---|---|
| Crystal grow | Temperature: 289 K / Method: vapor diffusion, sitting drop / pH: 7 Details: 0.2 M calcium acetate hydrate, 0.1 M Tris, pH 7.0, 20% PEG3000 |
-Data collection
| Diffraction | Mean temperature: 100 K / Serial crystal experiment: N |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  APS APS  / Beamline: 21-ID-G / Wavelength: 0.97856 Å / Beamline: 21-ID-G / Wavelength: 0.97856 Å |
| Detector | Type: MARMOSAIC 300 mm CCD / Detector: CCD / Date: Apr 23, 2017 |
| Radiation | Monochromator: double crystal Si(111) / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.97856 Å / Relative weight: 1 |
| Reflection | Resolution: 2.5→40 Å / Num. obs: 79421 / % possible obs: 98.8 % / Redundancy: 4.9 % / Rmerge(I) obs: 0.075 / Rpim(I) all: 0.041 / Rrim(I) all: 0.098 / Net I/σ(I): 21.5 |
| Reflection shell | Resolution: 2.5→2.54 Å / Rmerge(I) obs: 0.638 / Num. unique obs: 3828 / Rpim(I) all: 0.364 / Rrim(I) all: 0.786 |
- Processing
Processing
| Software |
| ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 6pba Resolution: 2.5→40 Å / Cross valid method: FREE R-VALUE Stereochemistry target values: GEOSTD + MONOMER LIBRARY + CDL V1.2
| ||||||||||||||||
| Displacement parameters | Biso mean: 64.05 Å2 | ||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.5→40 Å
|
 Movie
Movie Controller
Controller












 PDBj
PDBj