+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 6pbf | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | ZINC9155420-bound TRPV5 in nanodiscs | ||||||||||||
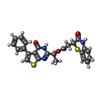
 Components Components | Transient receptor potential cation channel subfamily V member 5 | ||||||||||||
 Keywords Keywords | TRANSPORT PROTEIN / TRPV5 / TRP Channel / Inhibitor / Calcium channel | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationregulation of urine volume / calcium ion import across plasma membrane / calcium ion homeostasis / calcium ion transmembrane transport / calcium channel activity / calcium ion transport / protein homotetramerization / calmodulin binding / apical plasma membrane / metal ion binding ...regulation of urine volume / calcium ion import across plasma membrane / calcium ion homeostasis / calcium ion transmembrane transport / calcium channel activity / calcium ion transport / protein homotetramerization / calmodulin binding / apical plasma membrane / metal ion binding / identical protein binding / plasma membrane Similarity search - Function | ||||||||||||
| Biological species |  | ||||||||||||
| Method | ELECTRON MICROSCOPY / single particle reconstruction / cryo EM / Resolution: 4.2 Å | ||||||||||||
 Authors Authors | Hughes, T.E.T. / Rosario, J.S.D. / Kapoor, A. / Yazici, A.T. / Fluck, E.C. / Filizola, M. / Rohacs, T. / Moiseenkova-Bell, V.Y. | ||||||||||||
| Funding support |  United States, 3items United States, 3items
| ||||||||||||
 Citation Citation |  Journal: Elife / Year: 2019 Journal: Elife / Year: 2019Title: Structure-based characterization of novel TRPV5 inhibitors. Authors: Taylor Et Hughes / John Smith Del Rosario / Abhijeet Kapoor / Aysenur Torun Yazici / Yevgen Yudin / Edwin C Fluck / Marta Filizola / Tibor Rohacs / Vera Y Moiseenkova-Bell /  Abstract: Transient receptor potential vanilloid 5 (TRPV5) is a highly calcium selective ion channel that acts as the rate-limiting step of calcium reabsorption in the kidney. The lack of potent, specific ...Transient receptor potential vanilloid 5 (TRPV5) is a highly calcium selective ion channel that acts as the rate-limiting step of calcium reabsorption in the kidney. The lack of potent, specific modulators of TRPV5 has limited the ability to probe the contribution of TRPV5 in disease phenotypes such as hypercalcemia and nephrolithiasis. Here, we performed structure-based virtual screening (SBVS) at a previously identified TRPV5 inhibitor binding site coupled with electrophysiology screening and identified three novel inhibitors of TRPV5, one of which exhibits high affinity, and specificity for TRPV5 over other TRP channels, including its close homologue TRPV6. Cryo-electron microscopy of TRPV5 in the presence of the specific inhibitor and its parent compound revealed novel binding sites for this channel. Structural and functional analysis have allowed us to suggest a mechanism of action for the selective inhibition of TRPV5 and lay the groundwork for rational design of new classes of TRPV5 modulators. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  6pbf.cif.gz 6pbf.cif.gz | 412.4 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb6pbf.ent.gz pdb6pbf.ent.gz | 335 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  6pbf.json.gz 6pbf.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  6pbf_validation.pdf.gz 6pbf_validation.pdf.gz | 1.2 MB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  6pbf_full_validation.pdf.gz 6pbf_full_validation.pdf.gz | 1.4 MB | Display | |
| Data in XML |  6pbf_validation.xml.gz 6pbf_validation.xml.gz | 64.6 KB | Display | |
| Data in CIF |  6pbf_validation.cif.gz 6pbf_validation.cif.gz | 96.3 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/pb/6pbf https://data.pdbj.org/pub/pdb/validation_reports/pb/6pbf ftp://data.pdbj.org/pub/pdb/validation_reports/pb/6pbf ftp://data.pdbj.org/pub/pdb/validation_reports/pb/6pbf | HTTPS FTP |
-Related structure data
| Related structure data |  20292MC  6pbeC M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
| #1: Protein | Mass: 82899.656 Da / Num. of mol.: 4 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   #2: Chemical | ChemComp-O6V / ( Has ligand of interest | Y | |
|---|
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method: single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: ZINC9155420-bound TRPV5 in nanodiscs / Type: COMPLEX / Entity ID: #1 / Source: RECOMBINANT |
|---|---|
| Source (natural) | Organism:  |
| Source (recombinant) | Organism:  |
| Buffer solution | pH: 8 |
| Specimen | Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: YES |
| Specimen support | Details: unspecified |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TITAN KRIOS |
| Electron gun | Electron source:  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD |
| Image recording | Electron dose: 1 e/Å2 / Detector mode: SUPER-RESOLUTION / Film or detector model: GATAN K2 SUMMIT (4k x 4k) |
- Processing
Processing
| EM software |
| |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION | |||||||||||||||||||||||||||
| Symmetry | Point symmetry: C4 (4 fold cyclic) | |||||||||||||||||||||||||||
| 3D reconstruction | Resolution: 4.2 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 21802 / Symmetry type: POINT |
 Movie
Movie Controller
Controller














 PDBj
PDBj