[English] 日本語
 Yorodumi
Yorodumi- PDB-6mxt: Crystal structure of human beta2 adrenergic receptor bound to sal... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 6mxt | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Crystal structure of human beta2 adrenergic receptor bound to salmeterol and Nb71 | |||||||||
 Components Components |
| |||||||||
 Keywords Keywords | SIGNALING PROTEIN/HORMONE / G protein-coupled receptor / adrenergic receptor / asthma drug / active conformation / nanobody / SIGNALING PROTEIN-HORMONE complex / membrane protein | |||||||||
| Function / homology |  Function and homology information Function and homology informationpositive regulation of mini excitatory postsynaptic potential / beta2-adrenergic receptor activity / negative regulation of smooth muscle contraction / AMPA selective glutamate receptor signaling pathway / norepinephrine-epinephrine-mediated vasodilation involved in regulation of systemic arterial blood pressure / positive regulation of autophagosome maturation / heat generation / norepinephrine binding / Adrenoceptors / positive regulation of lipophagy ...positive regulation of mini excitatory postsynaptic potential / beta2-adrenergic receptor activity / negative regulation of smooth muscle contraction / AMPA selective glutamate receptor signaling pathway / norepinephrine-epinephrine-mediated vasodilation involved in regulation of systemic arterial blood pressure / positive regulation of autophagosome maturation / heat generation / norepinephrine binding / Adrenoceptors / positive regulation of lipophagy / negative regulation of G protein-coupled receptor signaling pathway / negative regulation of multicellular organism growth / response to psychosocial stress / endosome to lysosome transport / adrenergic receptor signaling pathway / diet induced thermogenesis / positive regulation of cAMP/PKA signal transduction / adenylate cyclase binding / smooth muscle contraction / bone resorption / potassium channel regulator activity / positive regulation of bone mineralization / brown fat cell differentiation / neuronal dense core vesicle / viral release from host cell by cytolysis / intercellular bridge / adenylate cyclase-activating adrenergic receptor signaling pathway / regulation of sodium ion transport / peptidoglycan catabolic process / receptor-mediated endocytosis / response to cold / clathrin-coated endocytic vesicle membrane / adenylate cyclase-modulating G protein-coupled receptor signaling pathway / cellular response to amyloid-beta / cell wall macromolecule catabolic process / lysozyme / mitotic spindle / lysozyme activity / Cargo recognition for clathrin-mediated endocytosis / positive regulation of cold-induced thermogenesis / amyloid-beta binding / Clathrin-mediated endocytosis / microtubule cytoskeleton / G alpha (s) signalling events / transcription by RNA polymerase II / host cell cytoplasm / early endosome / cell surface receptor signaling pathway / lysosome / receptor complex / positive regulation of MAPK cascade / endosome / endosome membrane / defense response to bacterium / Ub-specific processing proteases / cilium / ciliary basal body / apical plasma membrane / protein-containing complex binding / Golgi apparatus / protein homodimerization activity / positive regulation of transcription by RNA polymerase II / identical protein binding / nucleus / membrane / plasma membrane Similarity search - Function | |||||||||
| Biological species |  Enterobacteria phage T4 (virus) Enterobacteria phage T4 (virus) Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.95934213525 Å MOLECULAR REPLACEMENT / Resolution: 2.95934213525 Å | |||||||||
 Authors Authors | Masureel, M. / Zou, Y. / Picard, L.P. / van der Westhuizen, E. / Mahoney, J.P. / Rodrigues, J.P.G.L.M. / Mildorf, T.J. / Dror, R.O. / Shaw, D.E. / Bouvier, M. ...Masureel, M. / Zou, Y. / Picard, L.P. / van der Westhuizen, E. / Mahoney, J.P. / Rodrigues, J.P.G.L.M. / Mildorf, T.J. / Dror, R.O. / Shaw, D.E. / Bouvier, M. / Pardon, E. / Steyaert, J. / Sunahara, R.K. / Weis, W.I. / Zhang, C. / Kobilka, B.K. | |||||||||
| Funding support |  United States, 2items United States, 2items
| |||||||||
 Citation Citation |  Journal: Nat. Chem. Biol. / Year: 2018 Journal: Nat. Chem. Biol. / Year: 2018Title: Structural insights into binding specificity, efficacy and bias of a beta2AR partial agonist. Authors: Masureel, M. / Zou, Y. / Picard, L.P. / van der Westhuizen, E. / Mahoney, J.P. / Rodrigues, J.P.G.L.M. / Mildorf, T.J. / Dror, R.O. / Shaw, D.E. / Bouvier, M. / Pardon, E. / Steyaert, J. / ...Authors: Masureel, M. / Zou, Y. / Picard, L.P. / van der Westhuizen, E. / Mahoney, J.P. / Rodrigues, J.P.G.L.M. / Mildorf, T.J. / Dror, R.O. / Shaw, D.E. / Bouvier, M. / Pardon, E. / Steyaert, J. / Sunahara, R.K. / Weis, W.I. / Zhang, C. / Kobilka, B.K. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  6mxt.cif.gz 6mxt.cif.gz | 261.7 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb6mxt.ent.gz pdb6mxt.ent.gz | 196.7 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  6mxt.json.gz 6mxt.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  6mxt_validation.pdf.gz 6mxt_validation.pdf.gz | 1.2 MB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  6mxt_full_validation.pdf.gz 6mxt_full_validation.pdf.gz | 1.1 MB | Display | |
| Data in XML |  6mxt_validation.xml.gz 6mxt_validation.xml.gz | 22.1 KB | Display | |
| Data in CIF |  6mxt_validation.cif.gz 6mxt_validation.cif.gz | 29.5 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/mx/6mxt https://data.pdbj.org/pub/pdb/validation_reports/mx/6mxt ftp://data.pdbj.org/pub/pdb/validation_reports/mx/6mxt ftp://data.pdbj.org/pub/pdb/validation_reports/mx/6mxt | HTTPS FTP |
-Related structure data
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||||||
| Unit cell |
|
- Components
Components
-Protein / Antibody , 2 types, 2 molecules AN
| #1: Protein | Mass: 53528.066 Da / Num. of mol.: 1 Fragment: chimera of T4 lysozyme fused to human beta2 adrenergic receptor (UNP residues 29-234,263-365) Mutation: M96T, M98T, N187E Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Enterobacteria phage T4 (virus), (gene. exp.) Enterobacteria phage T4 (virus), (gene. exp.)  Homo sapiens (human) Homo sapiens (human)Gene: e, T4Tp126, ADRB2, ADRB2R, B2AR / Plasmid: pVL1392 / Production host:  References: UniProt: D9IEF7, UniProt: P07550, UniProt: P00720*PLUS, lysozyme |
|---|---|
| #2: Antibody | Mass: 13473.763 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   |
-Non-polymers , 8 types, 29 molecules 



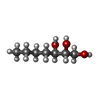










| #3: Chemical | ChemComp-K5Y / | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| #4: Chemical | ChemComp-MG / | ||||||||||
| #5: Chemical | | #6: Chemical | ChemComp-OLC / ( | #7: Chemical | ChemComp-HTO / | #8: Chemical | ChemComp-OLA / | #9: Chemical | ChemComp-P33 / | #10: Water | ChemComp-HOH / | |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 3.8 Å3/Da / Density % sol: 67.64 % |
|---|---|
| Crystal grow | Temperature: 293.15 K / Method: lipidic cubic phase / pH: 7.5 Details: 31-34% PEG400, 100 mM HEPES, pH 7.5, 1% 1,2,3-heptanetriol |
-Data collection
| Diffraction | Mean temperature: 77 K / Serial crystal experiment: N |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  APS APS  / Beamline: 23-ID-D / Wavelength: 0.987 Å / Beamline: 23-ID-D / Wavelength: 0.987 Å |
| Detector | Type: MAR CCD 130 mm / Detector: CCD / Date: Dec 18, 2012 |
| Radiation | Monochromator: Double crystal cryo-cooled Si(111) / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.987 Å / Relative weight: 1 |
| Reflection | Resolution: 2.95→30 Å / Num. obs: 18742 / % possible obs: 92.6 % / Redundancy: 3.3 % / Biso Wilson estimate: 52.7347764892 Å2 / Rmerge(I) obs: 0.167 / Net I/σ(I): 1.52 |
| Reflection shell | Resolution: 2.95→3.09 Å / Rmerge(I) obs: 0.525 / Mean I/σ(I) obs: 1.52 / Num. unique obs: 1012 / Χ2: 7.5 / % possible all: 75 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: PDB entries 4GBR & 4LDE Resolution: 2.95934213525→28.5196106168 Å / SU ML: 0.402084390742 / Cross valid method: FREE R-VALUE / σ(F): 1.38231122584 / Phase error: 24.3772282005
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 53.7137652891 Å2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.95934213525→28.5196106168 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
|
 Movie
Movie Controller
Controller














 PDBj
PDBj




















